
Write the principle behind Millikan's oil drop experiment.
Answer
586.8k+ views
Hint: The oil drop experiment was done by Robert A. Millikan and Harvey Fletcher in 1969 in order to calculate the elementary electric charge. The experiment took place in the Ryerson Physical Laboratory at the University of Chicago. Millikan received the Nobel Prize in Physics for this in 1992.
Complete step by step answer:
In 1909, Robert Millikan and Harvey Fletcher performed the oil drop experiment in order to determine the charge of an electron. By repeating the experiment, they proved that the charges were all multiples of a fundamental value. Using this apparatus, he was able to determine that the charge on an electron was $1.6\times {{10}^{-19}}C$. Professor Millikan has created so many innovations to improve the experiment. The droplets used are that of oil instead of water in order to reduce the tendency of the droplets to evaporate while the experiment is being done. It was later understood to the fact that Millikan's value of the viscosity of air was a little low. This experiment was based on the study of the motion of the uncharged oil drop in free fall because gravity and charged oil drop is kept in a uniform electric field. By keeping a uniform electric field, a charged oil drop can be developed to move up or down or even kept balanced in the field of view for a long time.
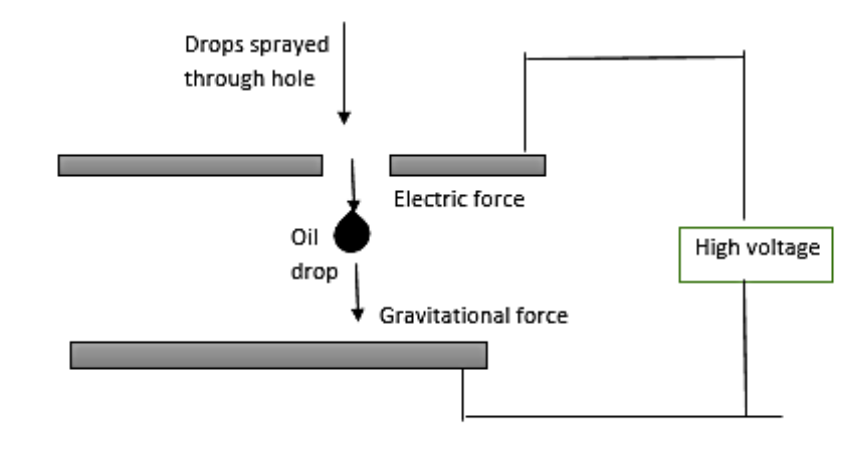
Note: The experiment was performed by observing tiny electrically charged droplets of oil located between two parallel metal surfaces, producing the plates of a capacitor. He used vacuum pump oil for his experiment. Millikan's experiment is special because it determined the charge on an electron. Millikan used a very easy apparatus in which he balanced the act of gravitational, electric, and drag forces.
Complete step by step answer:
In 1909, Robert Millikan and Harvey Fletcher performed the oil drop experiment in order to determine the charge of an electron. By repeating the experiment, they proved that the charges were all multiples of a fundamental value. Using this apparatus, he was able to determine that the charge on an electron was $1.6\times {{10}^{-19}}C$. Professor Millikan has created so many innovations to improve the experiment. The droplets used are that of oil instead of water in order to reduce the tendency of the droplets to evaporate while the experiment is being done. It was later understood to the fact that Millikan's value of the viscosity of air was a little low. This experiment was based on the study of the motion of the uncharged oil drop in free fall because gravity and charged oil drop is kept in a uniform electric field. By keeping a uniform electric field, a charged oil drop can be developed to move up or down or even kept balanced in the field of view for a long time.

Note: The experiment was performed by observing tiny electrically charged droplets of oil located between two parallel metal surfaces, producing the plates of a capacitor. He used vacuum pump oil for his experiment. Millikan's experiment is special because it determined the charge on an electron. Millikan used a very easy apparatus in which he balanced the act of gravitational, electric, and drag forces.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




