
Write the reactions involved in the following:
(a) Etard reaction
(b) Stephen reduction
Answer
585.9k+ views
Hint: Chemical reactions begin with reactants and end with products. Synthesis reactions bond reactants together, a process that requires energy, whereas decomposition reactions break the bonds within a reactant and thereby release energy. In exchange reactions, bonds are both broken and formed, and energy is exchanged
Complete step by step solution:
we have been given Etard and Stephen reaction:
So, The Etard reaction is an oxidation reaction, and the specific oxidizing agent the Etard reaction employs is chromyl chloride. Chromyl chloride is a chromium-based, mild oxidizing agent that is excellent at making aldehydes.
The reactions involved in Etard reaction is:
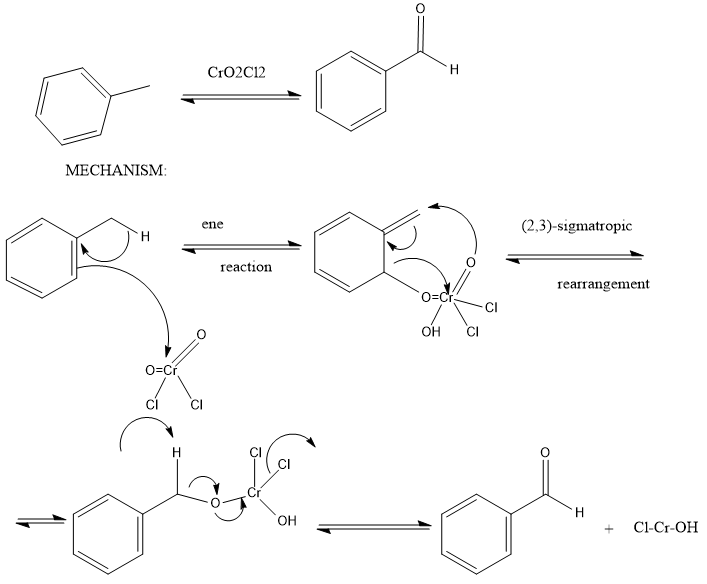
Now, Stephen reaction: Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen. This reaction involves the preparation of aldehydes from nitriles using tin (II) chloride, hydrochloric acid and quenching the resulting iminium salt with water. During the synthesis, ammonium chloride is also produced
The reaction involved in Stephen reaction is:

So, these are the reactions involving the Etard and Stephen reaction.
Note: When toluene is reacted with Chromyl Chloride then a chromium complex is formed (Etard Complex) whose hydrolysis gives Benzaldehyde. Here Chromyl Chloride is used to form an organic complex (Etard Complex) which can undergo hydrolysis to form the desired compound (in this case Benzaldehyde).
Complete step by step solution:
we have been given Etard and Stephen reaction:
So, The Etard reaction is an oxidation reaction, and the specific oxidizing agent the Etard reaction employs is chromyl chloride. Chromyl chloride is a chromium-based, mild oxidizing agent that is excellent at making aldehydes.
The reactions involved in Etard reaction is:

Now, Stephen reaction: Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen. This reaction involves the preparation of aldehydes from nitriles using tin (II) chloride, hydrochloric acid and quenching the resulting iminium salt with water. During the synthesis, ammonium chloride is also produced
The reaction involved in Stephen reaction is:

So, these are the reactions involving the Etard and Stephen reaction.
Note: When toluene is reacted with Chromyl Chloride then a chromium complex is formed (Etard Complex) whose hydrolysis gives Benzaldehyde. Here Chromyl Chloride is used to form an organic complex (Etard Complex) which can undergo hydrolysis to form the desired compound (in this case Benzaldehyde).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




