
Write the resonance structures of $CO_3^{2 - }$ and $HCO_3^ - $ .
Answer
583.5k+ views
Hint:The process of the delocalization of the $\pi - $ electrons in any compound which causes a lowering of the energy of the compound and thus, providing stability to it is known as resonance. The energy that is released from the compound while it undergoes resonance is known as resonance energy.
Complete step by step answer:
Under the complete framework of the valence bond theory, resonance is a simpler extension of the idea that the bonding between the atoms in a chemical species can be described with the help of a Lewis structure. For various chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. However, in some cases, more than one Lewis structure could be drawn, and experimental properties are inconsistent with any one structure. In order to address this type of situation, several contributing structures are considered together as an average, and the molecule is said to be represented by a resonance hybrid in which several Lewis structures are used collectively to describe its true structure.
The resonance structures of $CO_3^{2 - }$ is as follows:
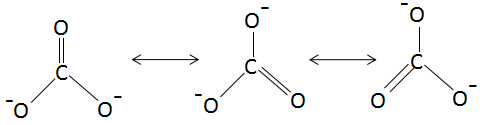
The resonance structures of $HCO_3^ - $ is as follows:
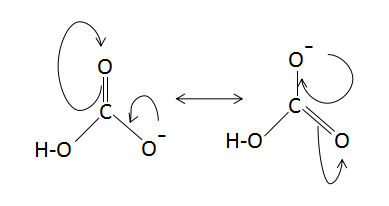
Note: The resonance hybrid represents the actual molecule as the average of the contributing structures, with bond lengths and partial charges taking on intermediate values as compared to those expected for the individual Lewis structures of the contributors, and if they were to exist as real chemical entities and not a hypothetical or imaginary entity.
Complete step by step answer:
Under the complete framework of the valence bond theory, resonance is a simpler extension of the idea that the bonding between the atoms in a chemical species can be described with the help of a Lewis structure. For various chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. However, in some cases, more than one Lewis structure could be drawn, and experimental properties are inconsistent with any one structure. In order to address this type of situation, several contributing structures are considered together as an average, and the molecule is said to be represented by a resonance hybrid in which several Lewis structures are used collectively to describe its true structure.
The resonance structures of $CO_3^{2 - }$ is as follows:

The resonance structures of $HCO_3^ - $ is as follows:

Note: The resonance hybrid represents the actual molecule as the average of the contributing structures, with bond lengths and partial charges taking on intermediate values as compared to those expected for the individual Lewis structures of the contributors, and if they were to exist as real chemical entities and not a hypothetical or imaginary entity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




