
Write the structure of aldehyde that yields


Answer
575.7k+ views
Hint: Aldehyde are those compounds which have an aldehydic group present in them and an aldehydic group is shown by CHO and all this compounds are ended with the suffix al i.e. named by ethanal.
Complete step by step answer: The compound given in the question is alcohol as it has OH group present in it and all compounds which have alcoholic groups are generally called alcohols and end with the suffix ol. Given compound is called 4-methylpent-2en-ol. This is generally the process known as reduction process.
In organic chemistry carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent. The main compounds which are in the category of carbonyl compounds are ketones, aldehydes, carboxylic acids, esters and acid halides. Carboxylic acids, esters and acid halides can be reduced to either aldehydes or after that they further reduced to primary alcohols and it generally depends on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation the alcohol can be further reduced and removed altogether. These reactions are produced with the help of metal hydrides of aluminum and lithium and these are known as reducing agents.
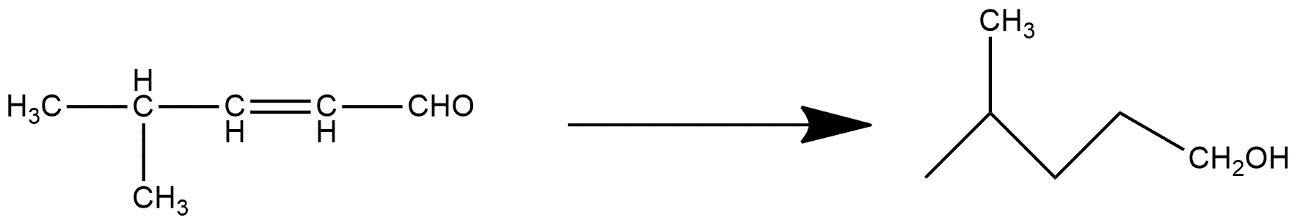
Hence we can say that 4-methylpent-2-ene-1al gives this compound.
Note: The reaction mechanism for metal hydride reduction is generally based on the concept of nucleophilic addition reaction of hydride to the carbonyl carbon. In some of the cases the alkali metal cation activates the carbonyl group by coordinating it with the carbonyl oxygen which generally enhances the electrophilicity of the carbonyl carbon.
Complete step by step answer: The compound given in the question is alcohol as it has OH group present in it and all compounds which have alcoholic groups are generally called alcohols and end with the suffix ol. Given compound is called 4-methylpent-2en-ol. This is generally the process known as reduction process.
In organic chemistry carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent. The main compounds which are in the category of carbonyl compounds are ketones, aldehydes, carboxylic acids, esters and acid halides. Carboxylic acids, esters and acid halides can be reduced to either aldehydes or after that they further reduced to primary alcohols and it generally depends on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation the alcohol can be further reduced and removed altogether. These reactions are produced with the help of metal hydrides of aluminum and lithium and these are known as reducing agents.
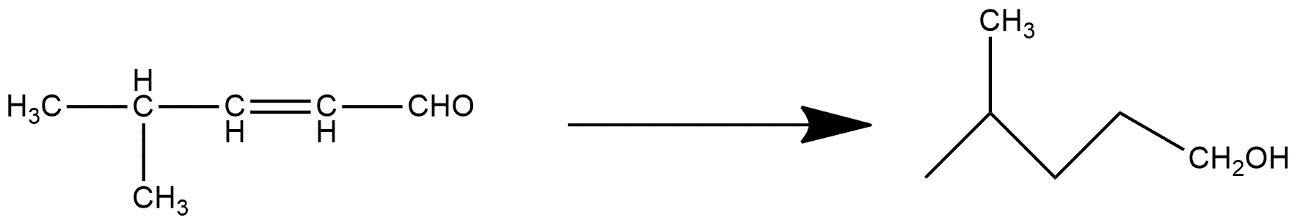
Hence we can say that 4-methylpent-2-ene-1al gives this compound.
Note: The reaction mechanism for metal hydride reduction is generally based on the concept of nucleophilic addition reaction of hydride to the carbonyl carbon. In some of the cases the alkali metal cation activates the carbonyl group by coordinating it with the carbonyl oxygen which generally enhances the electrophilicity of the carbonyl carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




