
Write the structure of cyclohexane.
Answer
582.6k+ views
Hint: Cyclohexane has molecular formulae of ${{C}_{6}}{{H}_{12}}$ and it has only secondary hydrogen atom. Cyclohexane forms a ring hence there is no $C{{H}_{3}}$ end there are only $C{{H}_{2}}$ ends.
Complete step by step answer:
Cyclohexane is a non-polar, colorless and flammable liquid with a detergent like odor. The easiest way using which we can draw the structure of cyclohexane is by simply drawing a hexagon. And in the hexagon each point depicts a fully saturated carbon atom with hydrogen atoms. When cyclohexane is depicted using a hexagon each carbon atom and each hydrogen atom in the structure appears the same.
Analysis of name of compound- Cyclohexane,
In this hex stands for six carbons it means that there are six carbons in the structure.
The suffix “ane” shows that the compound is saturated which means that there is only a single bond in the structure of the compound which means that the carbon are attached with each other through a single bond. And at last cyclo means there is a ring.
After the analysis of name of compound cyclohexane the structure is:
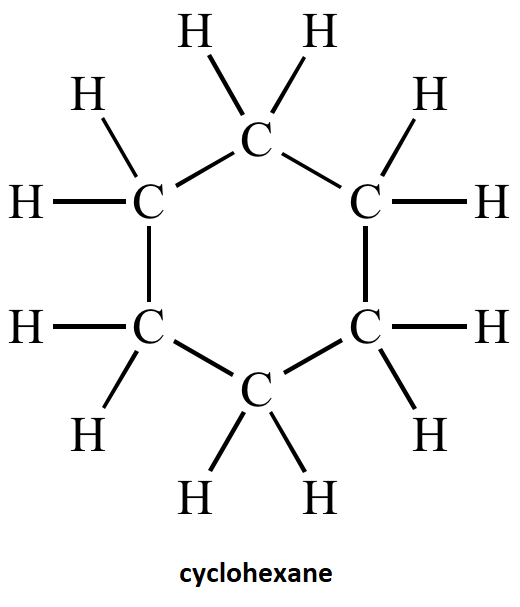
Note: Cyclohexane rings form several warped conformations to make the bond angles closer to the tetrahedral angles and also reduce the strain energy. Examples of some common conformation made by cyclohexane are the boat form, twist boat form, the chair form, half chair form. All these conformations of cyclohexane are named on the basis of shape that the cyclohexane molecule assumes in them. There is hydrogen- hydrogen interaction in these conformations.
Complete step by step answer:
Cyclohexane is a non-polar, colorless and flammable liquid with a detergent like odor. The easiest way using which we can draw the structure of cyclohexane is by simply drawing a hexagon. And in the hexagon each point depicts a fully saturated carbon atom with hydrogen atoms. When cyclohexane is depicted using a hexagon each carbon atom and each hydrogen atom in the structure appears the same.
Analysis of name of compound- Cyclohexane,
In this hex stands for six carbons it means that there are six carbons in the structure.
The suffix “ane” shows that the compound is saturated which means that there is only a single bond in the structure of the compound which means that the carbon are attached with each other through a single bond. And at last cyclo means there is a ring.
After the analysis of name of compound cyclohexane the structure is:

Note: Cyclohexane rings form several warped conformations to make the bond angles closer to the tetrahedral angles and also reduce the strain energy. Examples of some common conformation made by cyclohexane are the boat form, twist boat form, the chair form, half chair form. All these conformations of cyclohexane are named on the basis of shape that the cyclohexane molecule assumes in them. There is hydrogen- hydrogen interaction in these conformations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




