
Write the structure of D-erythrose?
Answer
574.8k+ views
Hint: Erythrose is a tetrose saccharide with the chemical formula ${C_4}{H_8}{O_4}$. It has one aldehyde group, and is part of the aldose family.
Complete answer:
Saccharide, is a group that includes sugars, starch, and cellulose commonly known as carbohydrates. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Carbohydrates are polyhydroxy aldehydes, ketones, alcohols, acids, their simple derivatives and their polymers having linkages of the acetyl type.
A Fischer projection is used to differentiate between L- and D- carbohydrates. On a Fischer projection of a monosaccharide, the penultimate carbon (alternatively, the last stereogenic carbon) of D sugars are depicted with hydrogen on the left and hydroxyl on the right. L sugars will be shown with the hydrogen on the right and the hydroxyl on the left. The D/L system (named after Latin dexter and laevus, right and left) names molecules by relating them to the molecule.
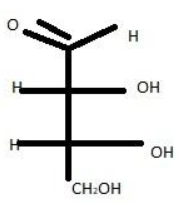
D-erythrose is the D-enantiomer of erythrose. It has a role as a plant metabolite. It is an enantiomer of a L-erythrose. Enantiomers are chiral molecules that are mirror images of one another. These molecules are non-superimposable on one another.
D-erythrose is a chiral molecule. $C-2$ and $C-3$ are stereocenters, both of which have the R configuration.
Note:
While assigning the D and L configurations of sugars, we directly look for the OH group of the bottom asymmetric carbon in the Fischer projection. If it's located on the right, we designate it with D, and if located on the left we designate it with L.
Complete answer:
Saccharide, is a group that includes sugars, starch, and cellulose commonly known as carbohydrates. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Carbohydrates are polyhydroxy aldehydes, ketones, alcohols, acids, their simple derivatives and their polymers having linkages of the acetyl type.
A Fischer projection is used to differentiate between L- and D- carbohydrates. On a Fischer projection of a monosaccharide, the penultimate carbon (alternatively, the last stereogenic carbon) of D sugars are depicted with hydrogen on the left and hydroxyl on the right. L sugars will be shown with the hydrogen on the right and the hydroxyl on the left. The D/L system (named after Latin dexter and laevus, right and left) names molecules by relating them to the molecule.

D-erythrose is the D-enantiomer of erythrose. It has a role as a plant metabolite. It is an enantiomer of a L-erythrose. Enantiomers are chiral molecules that are mirror images of one another. These molecules are non-superimposable on one another.
D-erythrose is a chiral molecule. $C-2$ and $C-3$ are stereocenters, both of which have the R configuration.
Note:
While assigning the D and L configurations of sugars, we directly look for the OH group of the bottom asymmetric carbon in the Fischer projection. If it's located on the right, we designate it with D, and if located on the left we designate it with L.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




