
Write the structure of neo-hexyl chloride?
Answer
540k+ views
Hint:To draw the structure of a molecule we should know how the nomenclature of the compounds are done. The prefix in the names refers to which group and which is the parent chain of the structure, how many carbon atoms are present etc.
Complete step-by-step answer:In the question, it is asked what will be the structure of neo-hexyl chloride.
Before going into the solution first we should be familiar with the name of the compound given what it refers to.
The name of the organic compound will give all the information about its structure. The parent chain of the organic molecule, the number of substituents attached and also the position at which the substituents are attached. The name of the compound also tells about the atom which is attached to the carbon chain as substituents.
According to the number of carbons in the parent chain the prefix changes:
If one C present then it will be methyl, if two C present then named as ethyl, for three C atoms, it will be propyl etc.
So here let us sketch the structure of the given molecule by following the IUPAC nomenclature rules set by IUPAC.
The compound has the name neo-hexyl chloride, we should first know what does the prefix neo-refers to. The structure with terminal tertiary group of C is called a neo structure or neo group. In the tertiary group the carbon which is of concern will be attached to three other alkyl chains by replacing three H atoms.
And in the structure the term hexyl refers that there are six carbons in the structure and the term chloride gives an idea that a Cl atom is also attached to the carbon chain.
So first draw the neo group, then attach that group with the number of carbon required to get a total of 6 carbon atoms. Since the position of the chlorine is not specified attach it in the first carbon.
And hence the structure is as follows:
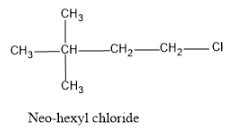
Note:The IUPAC name of neo hexyl chloride is 1-chloro-2,2-dimethylbutane, as the parent carbon chain is butane since there are four atoms and in the second carbon two methyl groups are attached and the Cl atom attached in the 1st carbon.
We should give the number for the substituents in such a way that the sum of the numbers of the substituents will yield less number possible.
Complete step-by-step answer:In the question, it is asked what will be the structure of neo-hexyl chloride.
Before going into the solution first we should be familiar with the name of the compound given what it refers to.
The name of the organic compound will give all the information about its structure. The parent chain of the organic molecule, the number of substituents attached and also the position at which the substituents are attached. The name of the compound also tells about the atom which is attached to the carbon chain as substituents.
According to the number of carbons in the parent chain the prefix changes:
If one C present then it will be methyl, if two C present then named as ethyl, for three C atoms, it will be propyl etc.
So here let us sketch the structure of the given molecule by following the IUPAC nomenclature rules set by IUPAC.
The compound has the name neo-hexyl chloride, we should first know what does the prefix neo-refers to. The structure with terminal tertiary group of C is called a neo structure or neo group. In the tertiary group the carbon which is of concern will be attached to three other alkyl chains by replacing three H atoms.
And in the structure the term hexyl refers that there are six carbons in the structure and the term chloride gives an idea that a Cl atom is also attached to the carbon chain.
So first draw the neo group, then attach that group with the number of carbon required to get a total of 6 carbon atoms. Since the position of the chlorine is not specified attach it in the first carbon.
And hence the structure is as follows:

Note:The IUPAC name of neo hexyl chloride is 1-chloro-2,2-dimethylbutane, as the parent carbon chain is butane since there are four atoms and in the second carbon two methyl groups are attached and the Cl atom attached in the 1st carbon.
We should give the number for the substituents in such a way that the sum of the numbers of the substituents will yield less number possible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




