
Write the structure of phenylmethanamine.
Answer
598.5k+ views
Hint: Benzylamine (sometimes abbreviated as $PhC{H_2}N{H_2}$ or $BnN{H_2}$) is an organismic organic compound simplified to molecular formula ${C_6}{H_5}C{H_2}N{H_2}$. This is composed of an amine functional group $N{H_2}$ and benzyl group ${C_6}{H_5}C{H_2}$. A common precursor in organic chemistry and used for the manufacture of many medicinal goods in industry.
Complete step by step solution:
> This waterless solution liquid is coloured. During Mercury-Atlas 6 Flight, NASA explorer John Glenn becomes the first Man to reach the Moon, utilizing hydrochloride powder. A variety of drugs, including alniditan, lacosamide, moxifloxacin, and nebivolol, are used in the producer of Benzylamine in manufacturing. This is often used in the production of HNIW, the industrial explosive superior to the old nitroamine-strong explosives such as HMX and RDX, although less durable. HNIW, with its less observed characteristics, such as the less conspicuous haze, is being used by the US Navy in weapon propellant applications, such as missiles.
> Benzylamine responds to N-benzylacetamide with acetyl chloride, an example of the originally mentioned Schotten – Baumann reaction in the eighties. The process is carried out in a two-component solvent environment (water and diethyl ether here), to avoid the by-product of the chloride from protonating and stopping the process from continuing in the water step (except at times with a neutralized base). Such requirements are also regarded as reaction conditions for Schotten-Baumann and are more commonly acceptable. As a description of the interfacial polymerization process of diamines utilizing a diacid chloride, this specific illustration is useful.
Phenylmethanamine has the structure as shown below-
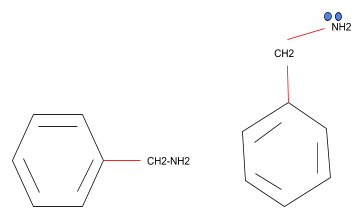
Note: Several approaches will generate benzylamine, with the main industrial route being benzyl chloride and ammonia reaction. The removal of benzonitrile and the removal of amination of benzaldehyde, all rendered by Raney nickel, also contributes to it.
Complete step by step solution:
> This waterless solution liquid is coloured. During Mercury-Atlas 6 Flight, NASA explorer John Glenn becomes the first Man to reach the Moon, utilizing hydrochloride powder. A variety of drugs, including alniditan, lacosamide, moxifloxacin, and nebivolol, are used in the producer of Benzylamine in manufacturing. This is often used in the production of HNIW, the industrial explosive superior to the old nitroamine-strong explosives such as HMX and RDX, although less durable. HNIW, with its less observed characteristics, such as the less conspicuous haze, is being used by the US Navy in weapon propellant applications, such as missiles.
> Benzylamine responds to N-benzylacetamide with acetyl chloride, an example of the originally mentioned Schotten – Baumann reaction in the eighties. The process is carried out in a two-component solvent environment (water and diethyl ether here), to avoid the by-product of the chloride from protonating and stopping the process from continuing in the water step (except at times with a neutralized base). Such requirements are also regarded as reaction conditions for Schotten-Baumann and are more commonly acceptable. As a description of the interfacial polymerization process of diamines utilizing a diacid chloride, this specific illustration is useful.
Phenylmethanamine has the structure as shown below-

Note: Several approaches will generate benzylamine, with the main industrial route being benzyl chloride and ammonia reaction. The removal of benzonitrile and the removal of amination of benzaldehyde, all rendered by Raney nickel, also contributes to it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




