
Write the structure of prop-2-en-1-amine.
Answer
581.1k+ views
Hint: First part represents the number of carbon atoms in the parent chain. The last part represents the functional group and ‘en’ represents the double bond.
Complete step by step answer:
The IUPAC rules used for the naming the alkenes are used to draw the structure of alkene.
The IUPAC rules for the naming of alkene are as follows:
- Determine the longest carbon chain and determine the substituents attached to the chain.
- If two same long chains are present then select the more substituted chain.
- Give the numbering to the carbon atoms of the chains in such a way so all the substituents get the lowest numbering.
- Arrange the substituents in alphabetical order with their position in the chain.
- If the same substituent is present more than one, then add ‘di’ for two, ‘tri’ for three, and so on.
- ‘prop’ indicates that the longest carbon chain is of three carbon atoms.
Draw the parent chain as follows:
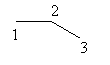
‘en’ suffix is used to show the presence of a double bond. 2 -en means the double bond is present at second carbon.
Draw the double bond at second carbon as follows:
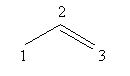
1-amine means that the amine functional group is present at first carbon.
Draw the amine group at first carbon as follows;
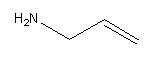
Therefore the structure of prop-2-en-1-amine is,
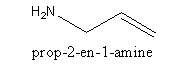
Note: In alkene the longest carbon chain includes both the carbon atoms having double bonds. The simple three-carbon alkene is known as propene where ‘prop’ indicates three carbons and ‘ene’ indicates a double bond. To specify the position of double bond the name is broken in two parts ‘prop’ and ‘ene’.
Complete step by step answer:
The IUPAC rules used for the naming the alkenes are used to draw the structure of alkene.
The IUPAC rules for the naming of alkene are as follows:
- Determine the longest carbon chain and determine the substituents attached to the chain.
- If two same long chains are present then select the more substituted chain.
- Give the numbering to the carbon atoms of the chains in such a way so all the substituents get the lowest numbering.
- Arrange the substituents in alphabetical order with their position in the chain.
- If the same substituent is present more than one, then add ‘di’ for two, ‘tri’ for three, and so on.
- ‘prop’ indicates that the longest carbon chain is of three carbon atoms.
Draw the parent chain as follows:

‘en’ suffix is used to show the presence of a double bond. 2 -en means the double bond is present at second carbon.
Draw the double bond at second carbon as follows:

1-amine means that the amine functional group is present at first carbon.
Draw the amine group at first carbon as follows;

Therefore the structure of prop-2-en-1-amine is,
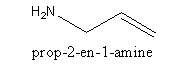
Note: In alkene the longest carbon chain includes both the carbon atoms having double bonds. The simple three-carbon alkene is known as propene where ‘prop’ indicates three carbons and ‘ene’ indicates a double bond. To specify the position of double bond the name is broken in two parts ‘prop’ and ‘ene’.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




