
write the structures of the following compounds:
(a) Prop-1-ene (b) 2, 3-dimethylbutane
(c) 2-methyl propane (d) 3-hexene
(e) prop-1-yne (f) 2-methyl prop-1-ene
(g) Alcohol with molecular formula ${{C}_{4}}{{H}_{10}}O$
Answer
532.8k+ views
Hint: Structure of any compound is the depiction of its IUPAC name. The IUPAC has set certain norms for the naming of organic compounds, these norms also affect the structures of organic compounds. Here are various structures of organic compounds.
Complete answer:
(a)Prop-1-ene: this compound has the molecular formula ${{C}_{3}}{{H}_{6}}$, as it is an alkene, therefore a double bond is present in propene. The structure is,
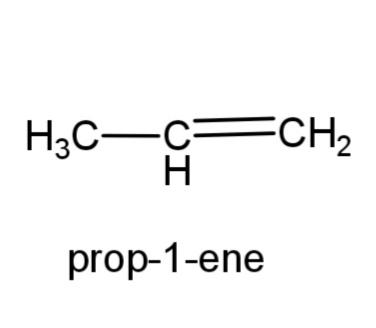
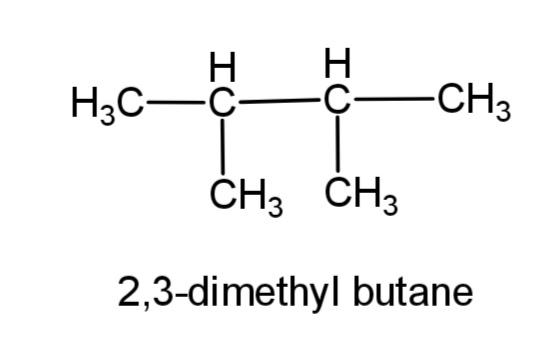
(b) 2, 3-dimethylbutane- this compound consists of a butane carbon chain with methyl group at 2 and 3 carbons. The structure is,
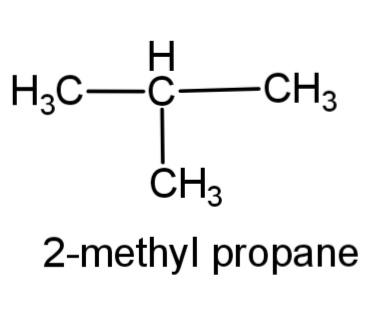
(c) 2-methyl propane – this compound consists of a 3 carbon propane chain with methyl group at carbon-2. The structure is,
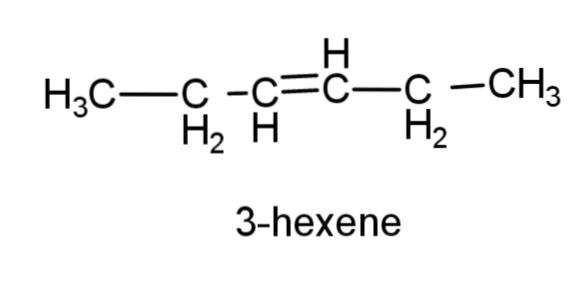
(d) 3-hexene – this compound consist of a 6 carbon chain, with a double bond at carbon 3, the structure is,
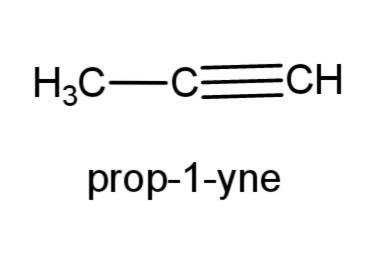
(e) prop-1-yne- this compound is an alkyne with triple bond at carbon 1, the structure is,
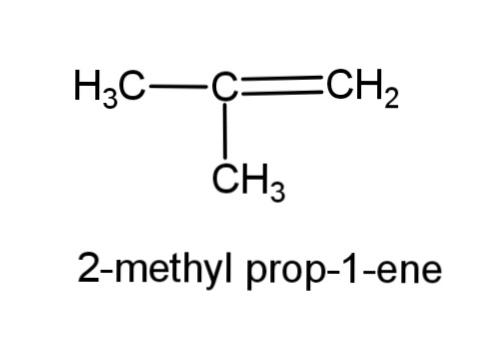
(f) 2-methyl prop-1-ene- this compound consist of a propene, with a methyl group at carbon-2, the structure is,
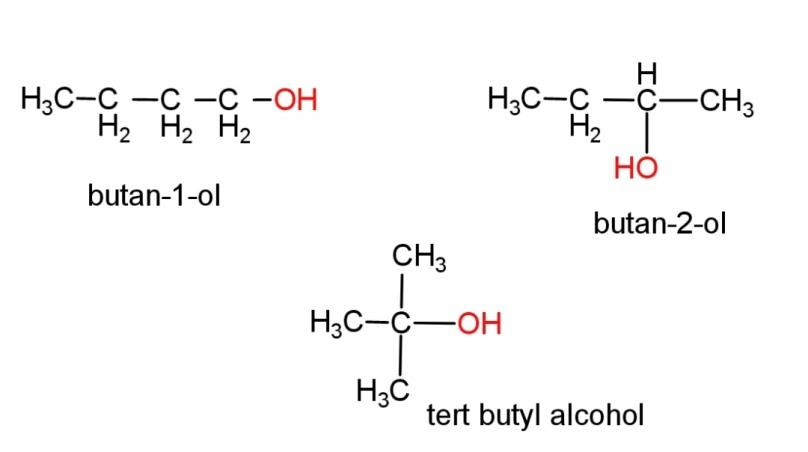
(g) Alcohol with molecular formula ${{C}_{4}}{{H}_{10}}O$ - there can be 3 different structures of alcohol with this molecular formula, the structures are,
Hence, all the structures have been shown above.
Note:
butan-2-ol consist of a chiral center, where all the 4 attached groups on the carbon atom are different. The chiral carbon is the second carbon. Chirality in a molecule results in optical rotation of the molecule with plane polarized light (PPL).
Complete answer:
(a)Prop-1-ene: this compound has the molecular formula ${{C}_{3}}{{H}_{6}}$, as it is an alkene, therefore a double bond is present in propene. The structure is,

(b) 2, 3-dimethylbutane- this compound consists of a butane carbon chain with methyl group at 2 and 3 carbons. The structure is,

(c) 2-methyl propane – this compound consists of a 3 carbon propane chain with methyl group at carbon-2. The structure is,

(d) 3-hexene – this compound consist of a 6 carbon chain, with a double bond at carbon 3, the structure is,

(e) prop-1-yne- this compound is an alkyne with triple bond at carbon 1, the structure is,

(f) 2-methyl prop-1-ene- this compound consist of a propene, with a methyl group at carbon-2, the structure is,

(g) Alcohol with molecular formula ${{C}_{4}}{{H}_{10}}O$ - there can be 3 different structures of alcohol with this molecular formula, the structures are,

Hence, all the structures have been shown above.
Note:
butan-2-ol consist of a chiral center, where all the 4 attached groups on the carbon atom are different. The chiral carbon is the second carbon. Chirality in a molecule results in optical rotation of the molecule with plane polarized light (PPL).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




