
Write the type of reaction,
Ethene is burnt in the presence of oxygen to form carbon dioxide , water and releases heat and light.
(a) endothermic reaction
(b) combustion reaction
(c) redox reaction
(d) esterification
Answer
566.7k+ views
Hint: Ethene or ethylene, a double bond containing carbon compound and member of the alkene family when subjected to the combustion breaks down into the carbon dioxide and water. Now you can easily identify the above reaction type of ethene molecule.
Complete step by step answer:
Ethene is a member belonging to the family of the alkene which consists of the double bond and from the name it is clear that it has two carbon atoms (eth=2) and thus, have the molecular formula as,${{\text{C}}_{2}}{{\text{H}}_{2}}$ and its common name is ethylene and is represented by the structure as:
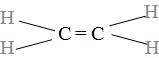
Ethene when reacts with the excess of oxygen it undergoes the combustion reaction( are those reactions in which the oxygen when made to reacts with the any substance, it produces carbon dioxide and water), the double bond present in the ethene breaks and thus, produces the water , carbon dioxide along with the evolution of the heat. The reaction occurs as:
\[\begin{matrix}
{{C}_{2}}{{H}_{4}} \\
ethene \\
\end{matrix}+\begin{matrix}
3{{O}_{2}} \\
oxygen \\
\end{matrix}\to \begin{matrix}
2C{{O}_{2}} \\
\begin{align}
& carbon \\
& dioxide \\
\end{align} \\
\end{matrix}+\begin{matrix}
2{{H}_{2}}O \\
water \\
\end{matrix}+Heat +light\]
So, thus the reaction of the ethene molecule with the oxygen is a combustion reaction’
So, the correct answer is “Option B”.
Note: Ethylene is used in the manufacture of polymers (these are the high molecular mass compounds which are made up of a large number of repeating units of monomers in a regular manner). Example: PVC (poly vinyl chloride), PET(poly ethylene terephthalate) and is used in packaging, construction industries and for packaging etc.
Complete step by step answer:
Ethene is a member belonging to the family of the alkene which consists of the double bond and from the name it is clear that it has two carbon atoms (eth=2) and thus, have the molecular formula as,${{\text{C}}_{2}}{{\text{H}}_{2}}$ and its common name is ethylene and is represented by the structure as:
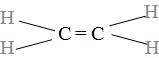
Ethene when reacts with the excess of oxygen it undergoes the combustion reaction( are those reactions in which the oxygen when made to reacts with the any substance, it produces carbon dioxide and water), the double bond present in the ethene breaks and thus, produces the water , carbon dioxide along with the evolution of the heat. The reaction occurs as:
\[\begin{matrix}
{{C}_{2}}{{H}_{4}} \\
ethene \\
\end{matrix}+\begin{matrix}
3{{O}_{2}} \\
oxygen \\
\end{matrix}\to \begin{matrix}
2C{{O}_{2}} \\
\begin{align}
& carbon \\
& dioxide \\
\end{align} \\
\end{matrix}+\begin{matrix}
2{{H}_{2}}O \\
water \\
\end{matrix}+Heat +light\]
So, thus the reaction of the ethene molecule with the oxygen is a combustion reaction’
So, the correct answer is “Option B”.
Note: Ethylene is used in the manufacture of polymers (these are the high molecular mass compounds which are made up of a large number of repeating units of monomers in a regular manner). Example: PVC (poly vinyl chloride), PET(poly ethylene terephthalate) and is used in packaging, construction industries and for packaging etc.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




