
Write the uses of Fullerene.
Answer
589.2k+ views
Hint: We know that carbon has many allotropes due to its valency. The known allotropes of carbon are diamond and graphite. Graphite is a soft material whereas diamond is hard. Few other allotropes of carbon are ball shaped structures known as buckminsterfullerene, sheet like structures known as graphene, nanotubes, nanoribbons and nanobuds. Nanocarbons consist of buckminsterfullerenes, carbon nanotubes, schwarzites and carbon nanobuds.
Complete step by step answer:
We know that the valency of carbon is four, and hence it has the ability to form many allotropes with various shapes. One of the allotrope of carbon is fullerene.
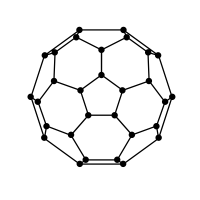
We can define the structure of fullerene as a molecule, which contains carbon atoms linked by single and double bonds to form, closed (or) semi closed mesh with five or seven atom-fused rings. Hollow sphere, tube, ellipsoid are some shapes of fullerene.
Buckminsterfullerene is one of the members of the fullerene family. The closed fullerene specially ${C_{60}}$ is referred to as buckyballs since it resembles soccer balls (footballs).
We can call cylindrical fullerenes as carbon nanotubes (or) buckytubes. Fullerite is the name given to the bulk solid shape of pure (or) mixed fullerene.
We can write the empirical formula of fullerene as ${C_n}$, where n indicated the number of atoms of carbon.
Buckminsterfullerene has twenty hexagons and twelve pentagons in their structure and each carbon contains three bonds.
We can get a violet solution, when buckminsterfullerene is dissolved in solvents of hydrocarbons. Buckminsterfullerene appears as black solid.
We can use fullerene in medical and tumor research. In the field of medicine, fullerene is used,
1.To light-activated antimicrobial agents
2.To target cancer cells such as antimicrobial agents
Buckminsterfullerene is used in drug delivery systems, in lubricants and as a catalyst.
We can use ${C_{60}}$ based films for photovoltaic applications.
Note:
Fullerenes are slightly soluble in solvents such as toluene, benzene, decane and carbon disulfide, whereas it is insoluble in water. Fullerenes have lubricating properties. ${C_{60}}$ is a soft material. Fullerenes have the ability to donate and accept electrons therefore; they can be used in batteries and electronic devices. In the form of carbon nanotubes, they can be used as materials for reinforcing composite.
Complete step by step answer:
We know that the valency of carbon is four, and hence it has the ability to form many allotropes with various shapes. One of the allotrope of carbon is fullerene.
We can define the structure of fullerene as a molecule, which contains carbon atoms linked by single and double bonds to form, closed (or) semi closed mesh with five or seven atom-fused rings. Hollow sphere, tube, ellipsoid are some shapes of fullerene.
Buckminsterfullerene is one of the members of the fullerene family. The closed fullerene specially ${C_{60}}$ is referred to as buckyballs since it resembles soccer balls (footballs).
We can call cylindrical fullerenes as carbon nanotubes (or) buckytubes. Fullerite is the name given to the bulk solid shape of pure (or) mixed fullerene.
We can write the empirical formula of fullerene as ${C_n}$, where n indicated the number of atoms of carbon.
Buckminsterfullerene has twenty hexagons and twelve pentagons in their structure and each carbon contains three bonds.
We can get a violet solution, when buckminsterfullerene is dissolved in solvents of hydrocarbons. Buckminsterfullerene appears as black solid.
We can use fullerene in medical and tumor research. In the field of medicine, fullerene is used,
1.To light-activated antimicrobial agents
2.To target cancer cells such as antimicrobial agents
Buckminsterfullerene is used in drug delivery systems, in lubricants and as a catalyst.
We can use ${C_{60}}$ based films for photovoltaic applications.

Note:
Fullerenes are slightly soluble in solvents such as toluene, benzene, decane and carbon disulfide, whereas it is insoluble in water. Fullerenes have lubricating properties. ${C_{60}}$ is a soft material. Fullerenes have the ability to donate and accept electrons therefore; they can be used in batteries and electronic devices. In the form of carbon nanotubes, they can be used as materials for reinforcing composite.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




