
Zeise’s salt is:
[A]$Fe{{\left( {{n}^{5}}-{{C}_{5}}{{H}_{5}} \right)}_{2}}$
[B]$Cr{{\left( {{n}^{6}}-{{C}_{6}}{{H}_{6}} \right)}_{2}}$
[C]$K\left[ Pt\left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)C{{l}_{3}} \right]$
[D]$K\left[ Pt{{\left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)}_{2}}C{{l}_{2}} \right]$
Answer
591k+ views
Hint: Zeise salt is the common name for potassium trichloro(ethylene)palatinate(II)hydrate. The anionic form of the zeise’s salt contains an ${{n}^{2}}$ethylene ligand.
Complete answer:
We find Zeise’s salt commercially in its hydrate form but anion is also stable and exists in air.
Zeise’s salt is named after its discoverer William Christopher Zeise. This salt is of historical importance as it is the first example of a transition metal alkene complex in organometallic chemistry.
Here we use the transition metal, platinum for the preparation of this salt. When platinum tetrachloride reacts with ethanol, it forms an adduct. If we add potassium chloride to this adduct, we obtain Zeise’s salt. We can write the reaction as-
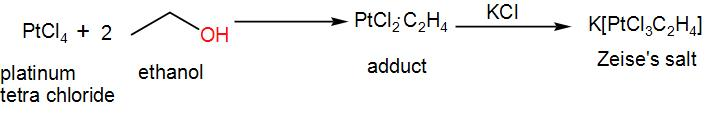
This coordination complex is stable. It is present in crystalline form and the crystals appear yellow or orange in colour.
However, in air the anion of this complex contains an ${{n}^{2}}$-ethylene ligand and the geometry of this anion is square planar. Its structure is-
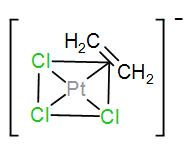
Here, as we can see in the structure, the carbon-carbon double bond is almost perpendicular to the plane of platinum chloride.
We can also prepare the hydrated Zeise’s salt by the reaction of potassium tetrachloroplatinate with ethylene and in presence of a catalytic amount of tin(II) chloride.
As we can tell from the above discussion that the formula of Zeise’s salt is $K\left[ Pt\left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)C{{l}_{3}} \right]$
Therefore, the correct answer is option [C] $K\left[ Pt\left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)C{{l}_{3}} \right]$.
Note: There is a model in organometallic chemistry named Dewar-Chatt-Duncanson model which explains how the metal, platinum (in this case) is co-ordinated to the carbon-carbon double bond.
Another compound was derived from the Zeise’s salt by eliminating potassium chloride and then dimerization. This compound was names Zeise’s dimer and its formula is${{\left[ \left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)PtC{{l}_{2}} \right]}_{2}}$.
Complete answer:
We find Zeise’s salt commercially in its hydrate form but anion is also stable and exists in air.
Zeise’s salt is named after its discoverer William Christopher Zeise. This salt is of historical importance as it is the first example of a transition metal alkene complex in organometallic chemistry.
Here we use the transition metal, platinum for the preparation of this salt. When platinum tetrachloride reacts with ethanol, it forms an adduct. If we add potassium chloride to this adduct, we obtain Zeise’s salt. We can write the reaction as-

This coordination complex is stable. It is present in crystalline form and the crystals appear yellow or orange in colour.
However, in air the anion of this complex contains an ${{n}^{2}}$-ethylene ligand and the geometry of this anion is square planar. Its structure is-

Here, as we can see in the structure, the carbon-carbon double bond is almost perpendicular to the plane of platinum chloride.
We can also prepare the hydrated Zeise’s salt by the reaction of potassium tetrachloroplatinate with ethylene and in presence of a catalytic amount of tin(II) chloride.
As we can tell from the above discussion that the formula of Zeise’s salt is $K\left[ Pt\left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)C{{l}_{3}} \right]$
Therefore, the correct answer is option [C] $K\left[ Pt\left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)C{{l}_{3}} \right]$.
Note: There is a model in organometallic chemistry named Dewar-Chatt-Duncanson model which explains how the metal, platinum (in this case) is co-ordinated to the carbon-carbon double bond.
Another compound was derived from the Zeise’s salt by eliminating potassium chloride and then dimerization. This compound was names Zeise’s dimer and its formula is${{\left[ \left( {{n}^{2}}-{{C}_{2}}{{H}_{4}} \right)PtC{{l}_{2}} \right]}_{2}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




