NCERT Exemplar for Class 12 Chemistry - Coordination Compounds - Free PDF Download
Free PDF download of NCERT Exemplar for Class 12 Chemistry Chapter 9 - Coordination Compounds solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 9 - Coordination Compounds exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations.
Coordination Compounds is one of the most important Chapters in Class 12. Students need to be in a position where they are clear with the basics that they learned in Class 11. The entire Chapter is an extension of what you have learned in Class 11. It takes a lot of similar concepts from the same topic and adds to the knowledge from the Chapter studied in Class 11.
Students must have a good idea of how the basic theory and core idea of the Chapter in Class 11 works. Only then they will be able to answer the questions in this Chapter. Class 12 Chemistry is dependent a lot on Inorganic Chemistry. Coordination Compounds being one of the most important Chapters of the subject can give you a lot of marks.
Access NCERT Exemplar Solutions for Class 12 Chemistry Chapter 9 – Coordination Compound
I. Multiple Choice Questions (Type-I)
1. Which of the following complexes formed by ${\text{C}}{{\text{u}}^{2 + }}$ ions are most stable?
(i) $\mathrm{Cu}^{2+}+4 \mathrm{NH}_{3} \rightleftharpoons\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}, \log \mathrm{K}=11.6$
(ii) $\mathrm{Cu}^{2+}+4 \mathrm{CN}_{-} \rightleftharpoons\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]^{2-}, \log \mathrm{K}=27.3$
(iii) $\mathrm{Cu}^{2+}+2 \mathrm{en} \rightleftharpoons\left[\mathrm{Cu}(\mathrm{en})_{2}\right]^{2+}, \log \mathrm{K}=15.4$
(iv) $\mathrm{Cu}^{2+}+4 \mathrm{H}_{2} \mathrm{O} \rightleftharpoons\left[\mathrm{Cu}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\right]^{2+}, \log \mathrm{K}=8.9$
Ans: Correct options: B
The bigger the value of constant, the better will be the stability.
Here, has the highest value of $logk$which corresponds to the highest value of $k$.
2. The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$, ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$.
A: ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$.
B: ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
C: ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
D: ${\left[ {{\text{Co(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$> ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$> ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$
Ans: Correct option: A
Strong field ligands have five degenerate energy levels, which means they have more energy separation than weak field ligands.
Here, ${\Delta{ E = }}\frac{{{\text{hc}}}}{{\lambda{ }}}$
${\Delta{ E}\alpha{ = }}\frac{{\text{1}}}{{\lambda{ }}}$
${\lambda{ }\alpha{ = }}\frac{{\text{1}}}{{{\Delta{ E}}}}$
The wavelength decreases as the energy separation rises.
3. When 0.1 mol ${\text{CoC}}{{\text{l}}_{\text{3}}}{{\text{(N}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{5}}}$ is treated with excess of ${\text{AgN}}{{\text{O}}_{\text{3}}}$, 0.2 mol of ${\text{AgCl}}$ are obtained. The conductivity of solution will correspond to
A: 1:3 electrolyte
B: 1:2 electrolyte
C: 1:1 electrolyte
D: 3:1 electrolyte
Ans: Correct option: B
When 0.1 mol ${\text{CoC}}{{\text{l}}_{\text{3}}}{{\text{(N}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{5}}}$ is treated with excess of ${\text{AgN}}{{\text{O}}_{\text{3}}}$, In the given reaction $\left[{{\text{CoC}}{{\text{l}}_{\text{3}}}{{{\text{(N}}{{\text{H}}_{\text{3}}}{\text{)}}}_{\text{5}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$
then electrolytic solution must contain
$\left[{{\text{CoC}}{{\text{l}}_{\text{3}}}{{{\text{(N}}{{\text{H}}_{\text{3}}}{\text{)}}}_{\text{5}}}} \right]{\text{C}}{{\text{l}}^{{\text{2 + }}}}$ and two ${\text{C}}{{\text{l}}^{\text{ - }}}$ ions.
Hence, it is 1:2 electrolyte.
4. When 1 mol $\left[{{\text{CrC}}{{\text{l}}_{\text{3}}}{{{\text{(}}{{\text{H}}_{\text{2}}}{\text{O)}}}_{\text{3}}}} \right]{\text{6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is treated with excess of ${\text{AgN}}{{\text{O}}_{\text{3}}}$, 3 mol of ${\text{AgCl}}$ are obtained. The formula of the complex is:
A: $\left[ {{\text{CrC}}{{\text{l}}_{\text{3}}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{3}}}{\text{ }}} \right]{\text{.3}}{{\text{H}}_{\text{2}}}{\text{O}}$
B: $\left[ {{\text{CrC}}{{\text{l}}_{\text{2}}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{4}}}} \right]{\text{Cl}}{\text{.2}}{{\text{H}}_{\text{2}}}{\text{O}}$
C: $\left[{{\text{CrC}}{{\text{l}}_{\text{2}}}{{{\text{(}}{{\text{H}}_{\text{2}}}{\text{O)}}}_{\text{5}}}}\right]{\text{C}}{{\text{l}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{O}}$
D: $\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}$
Ans: Correct option: D
When 1 mol $\left[{{\text{CrC}}{{\text{l}}_{\text{3}}}{{{\text{(}}{{\text{H}}_{\text{2}}}{\text{O)}}}_{\text{3}}}} \right]{\text{6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is treated with excess of ${\text{AgN}}{{\text{O}}_{\text{3}}}$, 3 mol of ${\text{AgCl}}$ is produced, i.e., $\left[{{\text{CrC}}{{\text{l}}_{\text{3}}}{{{\text{(}}{{\text{H}}_{\text{2}}}{\text{O)}}}_{\text{3}}}} \right]{\text{6}}{{\text{H}}_{\text{2}}}{\text{O}}$is dissociated in aqueous solution and all three chloride comes in solution.
$\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}} \to {\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O)6}}} \right]^{{\text{3 + }}}}{\text{ + 3C}}{{\text{l}}^{\text{ - }}}$
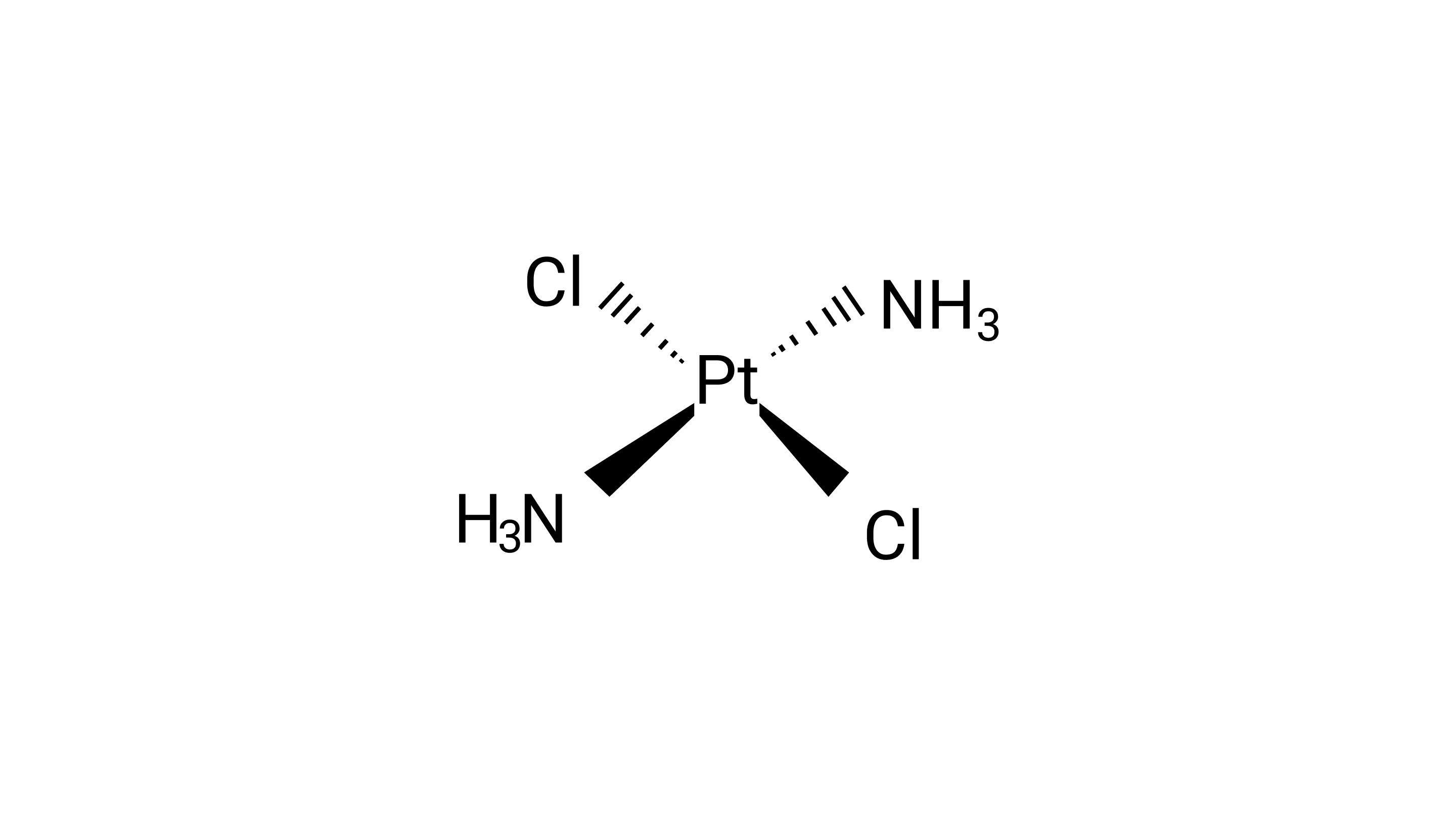
5: The correct IUPAC name of $\left[{{\text{Pt(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]$ is
(i) Diamminedichloridoplatinum (II)
(ii) Diamminedichloridoplatinum (IV)
(iii) Diamminedichloridoplatinum (0)
(iv) Dichloridodiammineplatinum (IV)
Ans: Correct option: A
Diamminedichloridoplatinum (II) is also known as azane
6. The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
A: $\left[ {{\text{Fe(CO}}{{\text{)}}_{\text{5}}}} \right]$
B: $\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]{{\text{3}}^{\text{ - }}}$
C: $\left[{{\text{Fe(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}} \right]{{\text{3}}^{\text{ - }}}$
D: $\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{{\text{3}}^{\text{ + }}}$
Ans: Correct option: C
The chelate effect is when a chelating ligand coordinates the stabilisation of a molecule. Any ligand that binds to metal forms a ring with 5 or 6 members, which is the most stable.
Here, ${\text{CO}}$, ${\text{CN}}$, ${{\text{H}}_{\text{2}}}{\text{O}}$ is monodentate ligand $\left[{{\text{Fe(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{3}}}} \right]{{\text{3}}^{\text{ - }}}$ is an oxalate ligand.
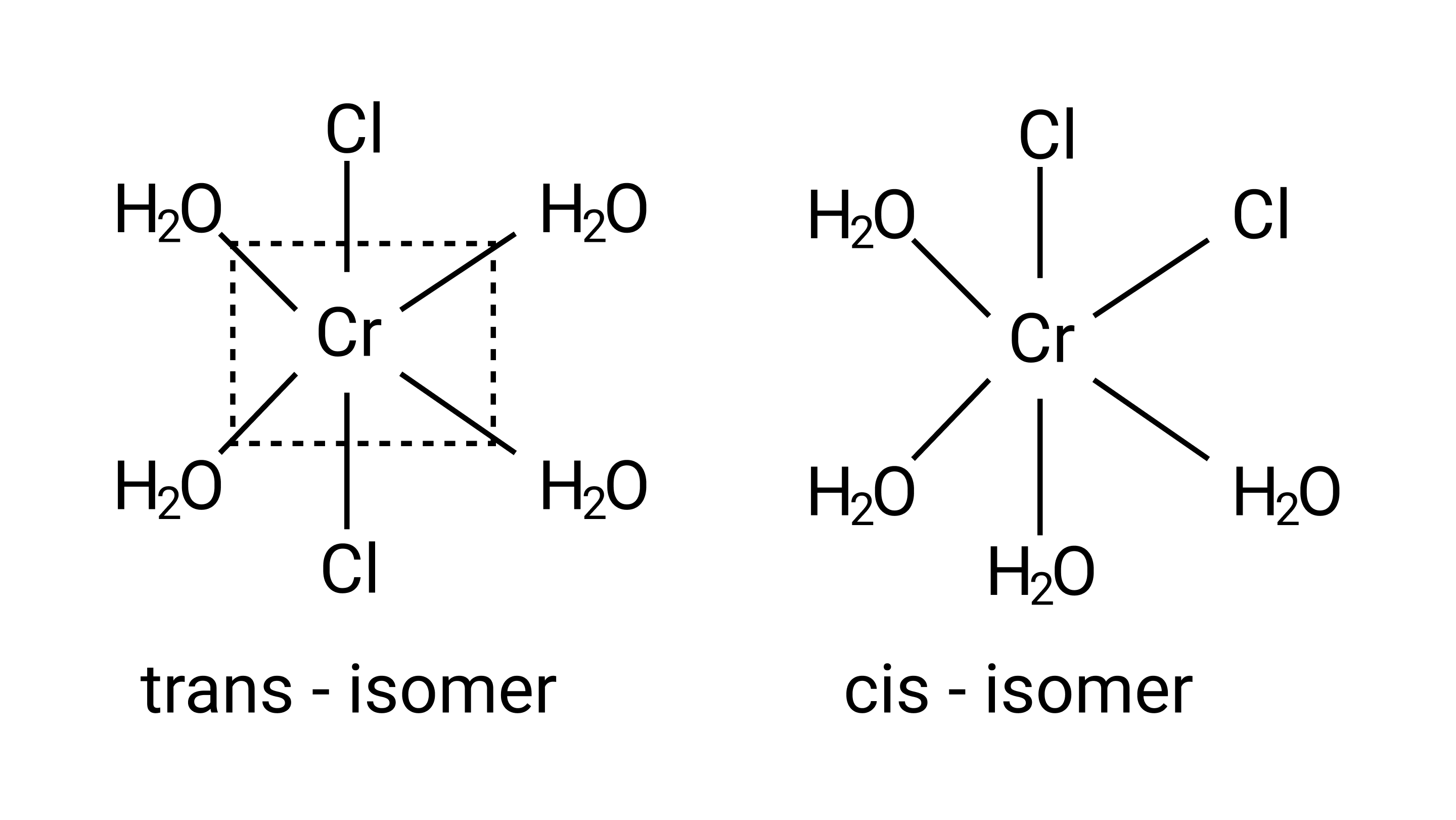
7: Indicate the complex ion which shows geometrical isomerism.
A: ${\left[{{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}$
B: $\left[ {{\text{Pt(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{Cl}}} \right]$
C: ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$
D: $\left[ {{\text{Co(CN)5}}} \right]{{\text{(NC)}}^{{\text{3 - }}}}$
Ans: Correct option: A
This is due to the complex's octahedral structure and (cis-trans) isomerism. Two ${\text{Cl}}$ ligands are next to each other in trans isomerism. Although these isomers have the same structure, they are not identical.
Possible isomers are:
8. The CFSE for octahedral ${\left[ {{\text{CoC}}{{\text{l}}_{\text{6}}}} \right]^{{\text{4 - }}}}$ is 18,000 $c{m^{ - 1}}$. The CFSE for tetrahedral ${\left[ {{\text{CoC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}$ will be
A: 18,000 ${\text{c}}{{\text{m}}^{ - 1}}$
B: 16,000 ${\text{c}}{{\text{m}}^{ - 1}}$
C: 8,000 ${\text{c}}{{\text{m}}^{ - 1}}$
D: 20,000 ${\text{c}}{{\text{m}}^{ - 1}}$
Ans: Correct option: A
CFSE for octahedral and tetrahedral complex is related as
$\Delta^{t}=\frac{4}{9} \Delta_{0}$
Where $\Delta_{0}=$ CFSE for octahedral complex
$\Delta_{0}=$ CFSE for tetrahedral complex
$\Delta_{0}=1800 \mathrm{~cm}^{-1}$
$\Delta_{t}=\frac{4}{9} \times 18000=8000\mathrm{~cm}^{-1}$
9. Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] are
(A) linkage isomers
(B) coordination isomers
(C) ionization isomers
(D) geometrical isomers
Ans: Correct option: (A)
Linkage isomerism arises in a coordination compound containing ambidentate ligand.
A simple example is by complexes containing the thiocyanate ligand, NCS-which may bind through the nitrogen to give M-NCS or through sulphur to give M-SCN.
10. The compounds $\left[{{\text{Co(S}}{{\text{O}}_{\text{4}}}{\text{)(N}}{{\text{H}}_{\text{3}}}{\text{)5}}} \right]{\text{Br}}$and $\left[ {{\text{Co(S}}{{\text{O}}_{\text{4}}}{\text{)(N}}{{\text{H}}_{\text{3}}}{\text{)5}}} \right]{\text{Cl}}$ represent
A: linkage isomerism
B: ionisation isomerism
C: coordination isomerism
D: no isomerism
Ans: Correct option: D
Two or more compounds with the same formula but distinct structures and characteristics are known as isomers.
So, in this case compounds
$\left[{{\text{Co(S}}{{\text{O}}_{\text{4}}}{\text{)(N}}{{\text{H}}_{\text{3}}}{\text{)5}}} \right]{\text{Br}}$ and $\left[{{\text{Co(S}}{{\text{O}}_{\text{4}}}{\text{)(N}}{{\text{H}}_{\text{3}}}{\text{)5}}} \right]{\text{Cl}}$ show no isomerism
11. A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent?
A: thiosulphato
B: oxalato
C glycinato
D: ethane-1,2-diamine
Ans: Correct option: A
A chelating agent binds to a single metal ion with two or more donor atoms. Oxaloto, Glycinato, and ethane-1,2-diamine all have two donor oxygen atoms. The gland Thiosulpato is ambidentate.
12. Which of the following species is not expected to be a ligand?
A: ${\text{NO}}$
B: ${\text{N}}{{\text{H}}^{{\text{4 + }}}}$
C: ${\text{N}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}$
D: ${\text{CO}}$
Correct option: B
Ligands are neutral ions that form a coordination complex with the central metal atom.
${\text{N}}{{\text{H}}^{{\text{4 + }}}}$ does not have a lone pair of electrons to give.
13. What kind of isomerism exists between $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}$ (violet) and $\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{Cl}_{2} \cdot \mathrm{H}_{2} \mathrm{O}$ (greyish-green)?
A: linkage isomerism
B: solvate isomerism
C: ionisation isomerism
D coordination isomerism
Ans: Correct option: B
Hydrate isomerism is isomerism in which water is used as a solvent.
Coordination compounds with the same composition but varying ligand connectivity are known as linkage isomers.
Solvate isomerism has the same composition as free solvent but distinct solvent ligand molecules.
Except for the ligand that swaps places with the anion, ionisation isomers are identical isomers.
Coordination isomers are coordination compounds with distinct metal and ligand compositions.
14. IUPAC name of
$\left[{{\text{Pt(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{Cl(N}}{{\text{O}}_{\text{2}}}{\text{)}}} \right]$ is
A: Platinum diaminechloronitrite
B: Chloronitrito-N-ammineplatinum (II)
C Diamminechloridonitrito-N-platinum (II)
D Diamminechloronitrito-N-platinate (II)
Ans: Correct option: C
$\left[{{\text{Pt(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{Cl(N}}{{\text{O}}_{\text{2}}}{\text{)}}} \right]$ is a neutral molecule. Ligands are arranged alphabetically.
So, ${\text{N}}{{\text{H}}_{\text{3}}}$ Diammine, chloride and the embedded linkage is nitrogen and last is the metal name.
II. Multiple Choice Questions (Type-II)
Note: In the following questions two or more options may be correct.
15. Atomic numbers of ${\text{Mn, Fe, and Co}}$ are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complex ions are diamagnetic?
A: ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$
B: ${\left[ {{\text{Mn(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
C: ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{4 - }}}}$
D: ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
Ans: Correct option: A and C
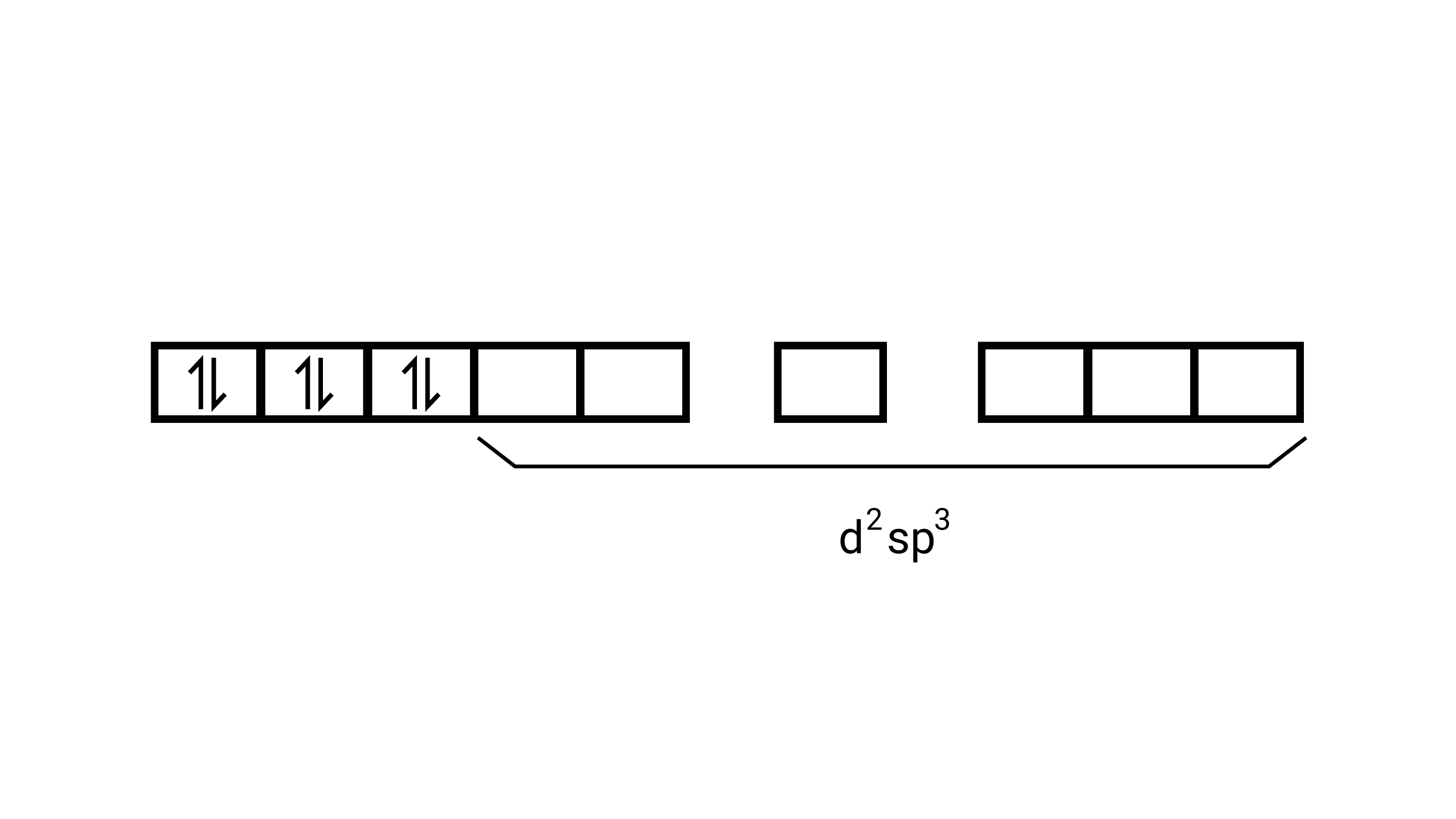
(i) Orbitals of $\mathrm{Co}^{3+}$ ion $\mathrm{d}^{2} \mathrm{sp}^{3}$ hybridised orbitals of $\mathrm{Co}^{3+}\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$ (inner orbital or low spin complex)
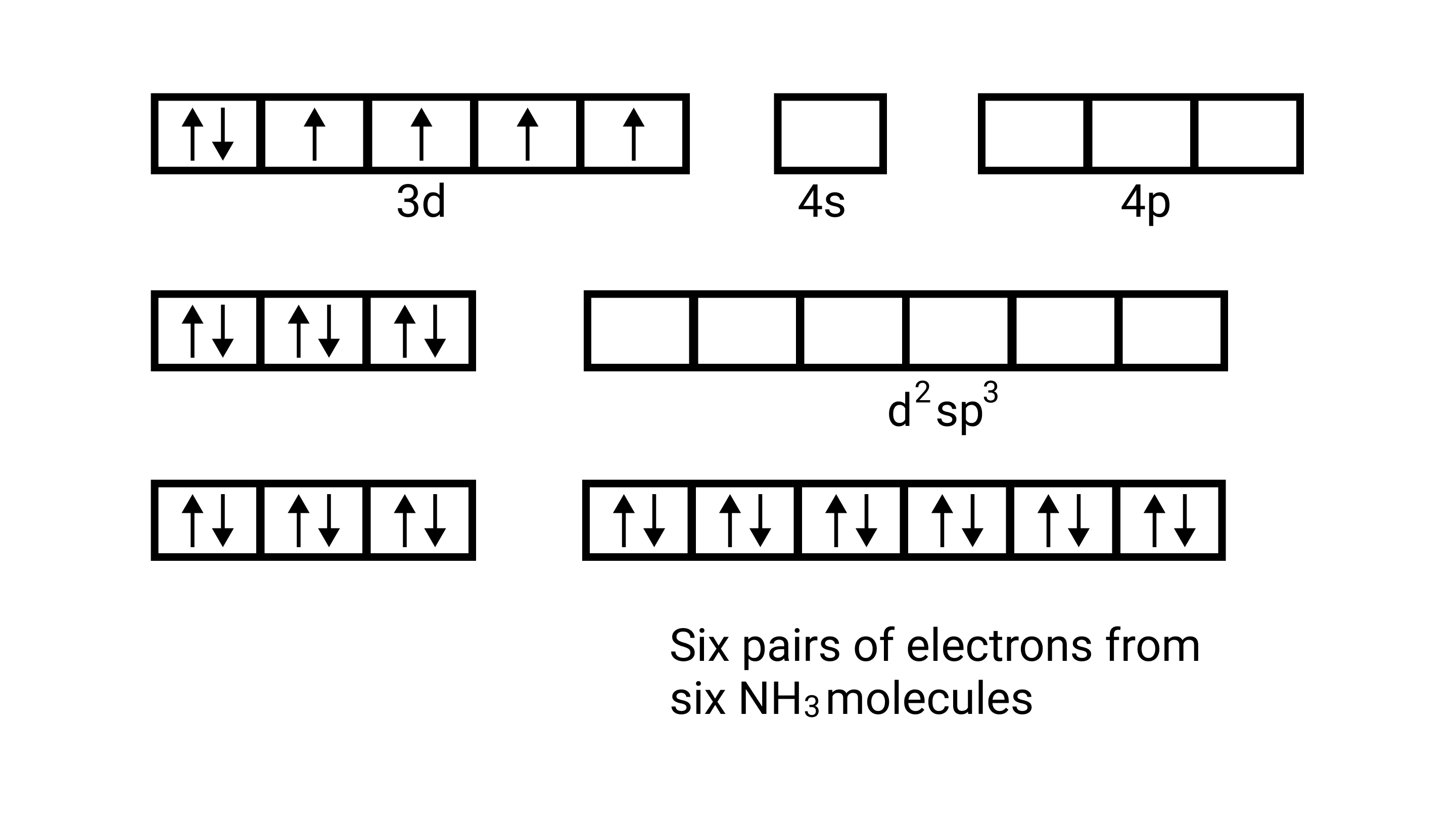
No. of unpaired electron $=0$
Magnetic property = diamagnetic , due to absence of unpaired electrons
(ii) Electronic configuration is $3 \mathrm{~d}^{6}$ orbitals of $\mathrm{Fe}^{2+}$ ion:
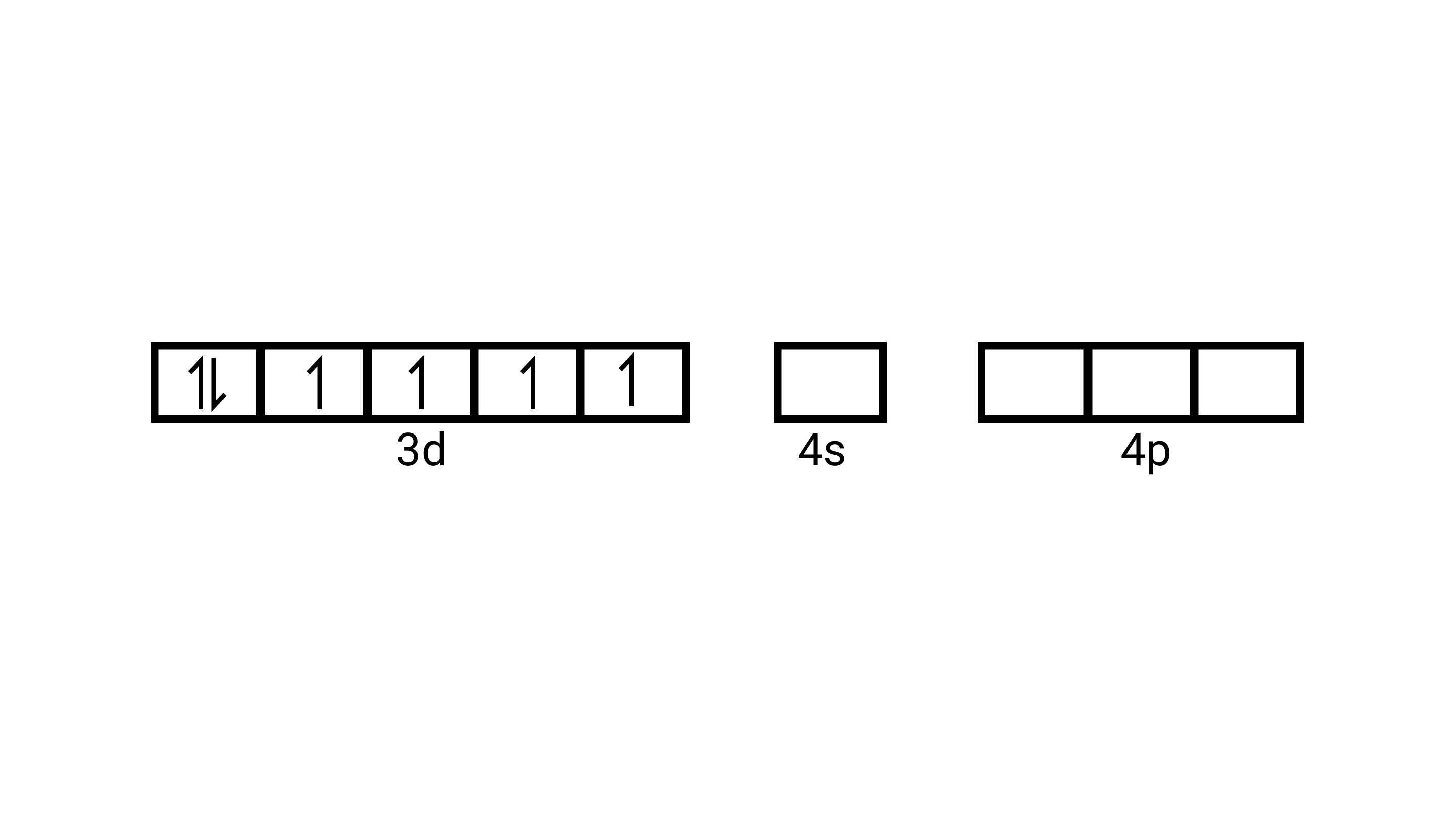
As $\mathrm{CN}^{-}$is a strong field ligand, it causes the pairing of the unpaired $3 d$ electrons. Since there are six ligands around the central metal ion, the most feasible hybridization $d^{2} s p^{3} \cdot d^{2} s p^{3}$ hybridized oribtals of $\mathrm{Fe}^{2+}$ are:
6 electron pairs from $\mathrm{CN}^{-}$ ions occupy the six hybrid $d^{2} s p^{3}$ orbitals. Then,
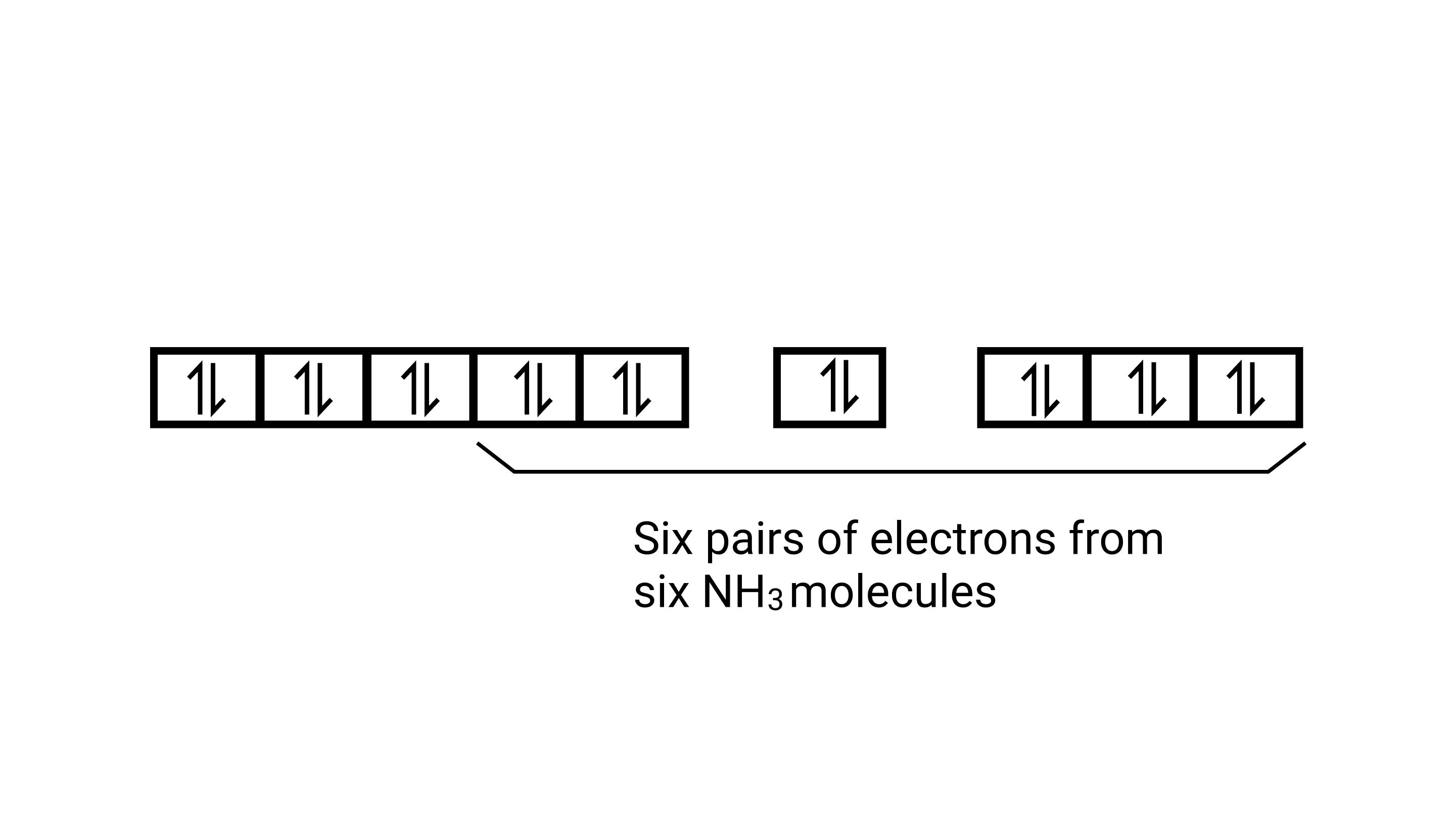
Hence, the geometry of the complex is octahedral and the complex is diamagnetic (as there are no unpaired electrons.
16. Atomic number of ${\text{Mn}}$, ${\text{Fe}}$, ${\text{Co}}$ and ${\text{Ni}}$ are 25, 26 27 and 28 respectively. Which of the following outer orbital octahedral complexes have same number of unpaired electrons?
A: ${\left[ {{\text{MnC}}{{\text{l}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
B: ${\left[ {{\text{Fe}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
C: ${\left[ {{\text{Co}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
D: ${\left[ {Ni{{(N{H_3})}_6}} \right]^{2 + }}$
Ans: Correct option: A and C
The outer octahedral complexes are weak ligands, number of pairing electrons and high spinning potential.
${\left[ {{\text{MnC}}{{\text{l}}_{\text{6}}}} \right]^{{\text{3 - }}}}$ - electronic configuration of ${\text{Mn = 3}}{{\text{d}}^{\text{5}}}{\text{,4}}{{\text{s}}^{\text{2}}}$. Shifting the valence electrons become
${\text{M}}{{\text{n}}^{{\text{3 + }}}}{\text{,3}}{{\text{d}}^{\text{4}}}$and number of unpaired electrons is 4.
${\left[ {{\text{Fe}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}$- electronic configuration of ${\text{Fe = 3}}{{\text{d}}^{\text{6}}}{\text{,4}}{{\text{s}}^{\text{2}}}$. Shifting the valence electrons become
${\text{F}}{{\text{e}}^{{\text{3 + }}}}{\text{,3}}{{\text{d}}^{\text{5}}}$and the number of unpaired electrons is 5.
${\left[ {{\text{Co}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}$electronic configuration of ${\text{Co = 3}}{{\text{d}}^7},{\text{4}}{{\text{s}}^2}$ shifting the valence electrons become
${\text{F}}{{\text{e}}^{{\text{3 + }}}}{\text{,3}}{{\text{d}}^{\text{6}}}$and the number of unpaired electrons is 4.
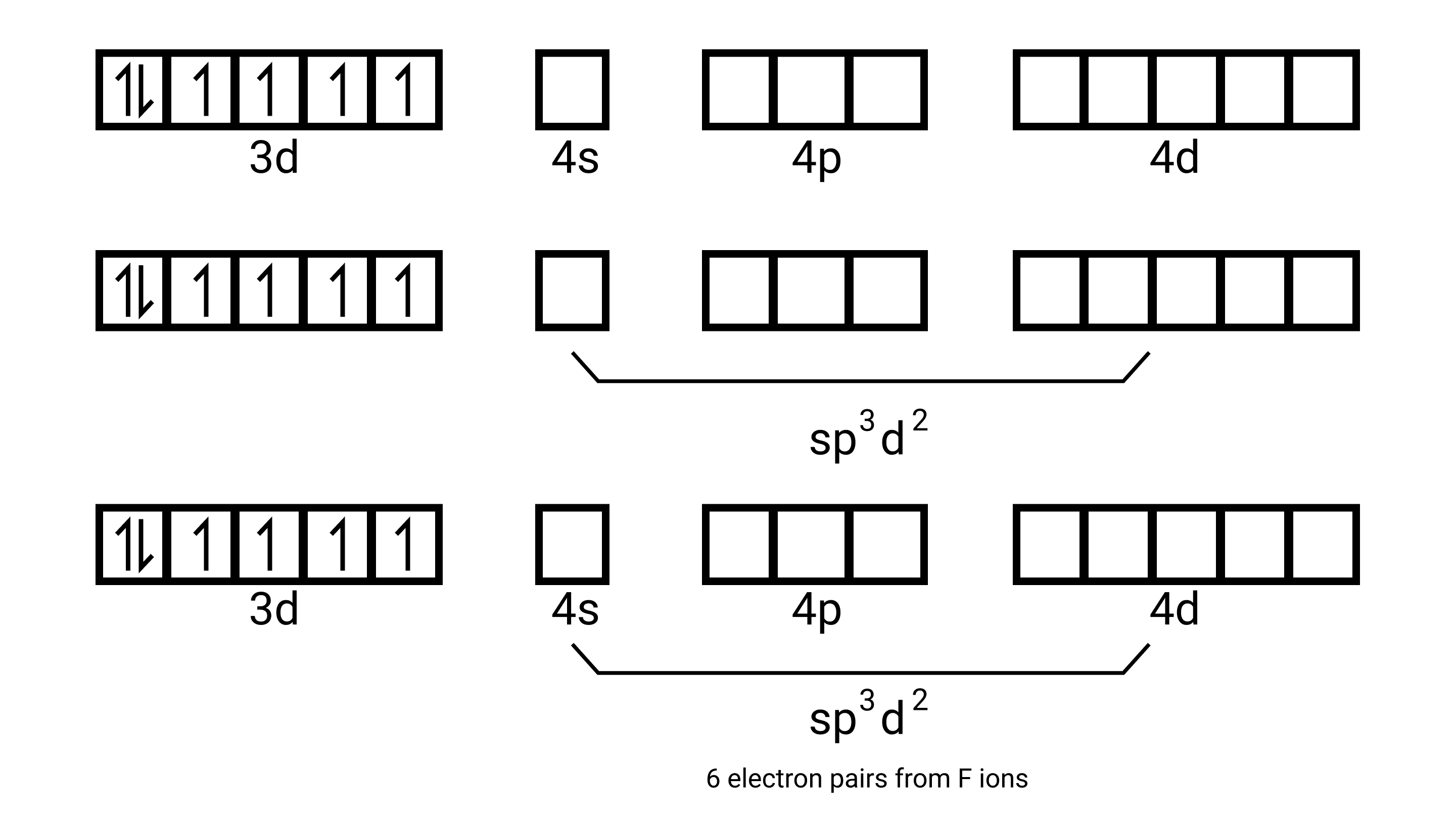
${\left[ {{\text{Ni(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}$- electronic configuration of ${\text{Ni = 3}}{{\text{d}}^{\text{8}}}{\text{,4}}{{\text{s}}^{\text{2}}}$. Shuffling the valence electrons become
${\text{N}}{{\text{i}}^{{\text{2 + }}}}{\text{,3}}{{\text{d}}^{\text{6}}}$ and the number of unpaired electrons is 2.
17. Which of the following options are correct for ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$ complex?
A: ${{\text{d}}^{\text{2}}}{\text{s}}{{\text{p}}^{\text{3}}}$ hybridisation
B: ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ hybridisation
C: paramagnetic
D: diamagnetic
Ans: Correct option: A and C
For answer at (A) Atomic number of Iron is 26 . In ferricyanide complex, iron is in oxidation state of $3^{+}$. The orbitals on the metal atom undergoes $\mathrm{d}^{2} \mathrm{sp}^{3}$ hybridizaion and hence the complex has octahedral shape.
Also it can be said that, $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]$ 3- has one unpaired electron which makes it weakiy paramagnetic. Therefore, option C is also correct.
18. An aqueous pink solution of cobalt (II) chloride changes to deep blue on addition of excess of ${\text{HCl}}$. This is because____________.
A: ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O)}}} \right]^{{\text{2 + }}}}$is transformed into ${\left[ {{\text{CoC}}{{\text{l}}^{\text{6}}}} \right]^{{\text{4 - }}}}$
B: ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}$is transformed into ${\left[ {{\text{CoC}}{{\text{l}}^{\text{4}}}} \right]^{{\text{2 - }}}}$
C: tetrahedral complexes have smaller crystal field splitting than octahedral complexes.
D: tetrahedral complexes have larger crystal field splitting than octahedral complex.
Ans: Correct option: B and C
${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}$ is increased when excess of ${\text{HCl}}$ is added. Tetrahedral complexes have smaller crystal field splitting than octahedral complexes because ${\Delta{ t = }}\frac{{\text{4}}}{{\text{9}}}{\Delta{ o}}$
19. Which of the following complexes are homoleptic?
A: ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$
B: ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}} \right]^{\text{ + }}}$
C: 4${\left[ {{\text{Ni(CN}}{{\text{)}}_{\text{4}}}} \right]^{{\text{2 - }}}}$
D: $\left[ {{\text{Ni(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]$
Ans: Correct options: A, B and C
A homoleptic complex is one that has only one species or group as a ligand.
Option A, B, and C each have only one ligand, but option D has two.
20. Which of the following complexes are heteroleptic?
A: ${\left[ {{\text{Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$
B: ${\left[ {{\text{Fe(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}{\text{Cl}}} \right]^{\text{ + }}}$
C: ${\left[ {{\text{Mn(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{4 - }}}}$
D: $\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]$
Ans: Correct option: B and D
Heteroleptic is a compound that contains many ligands of various sorts. ${\text{N}}{{\text{H}}_{\text{3}}}$ and
${\text{Cl}}$ as a ligand or also called as donor groups, is a heteroleptic complex.
21. Identify the optically active compounds from the following.
A: ${\left[ {{\text{Co(en}}{{\text{)}}_{\text{3}}}} \right]^{{\text{3 + }}}}$
B: ${\text{trans - }}{\left[ {{\text{Co(en}}{{\text{)}}_{\text{2}}}} \right]^{\text{ + }}}$
C: ${\text{cis - }}{\left[ {{\text{Co(en}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}$ $
D: $\left[ {{\text{Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{Cl}}} \right]$
Ans: Correct option: A and C
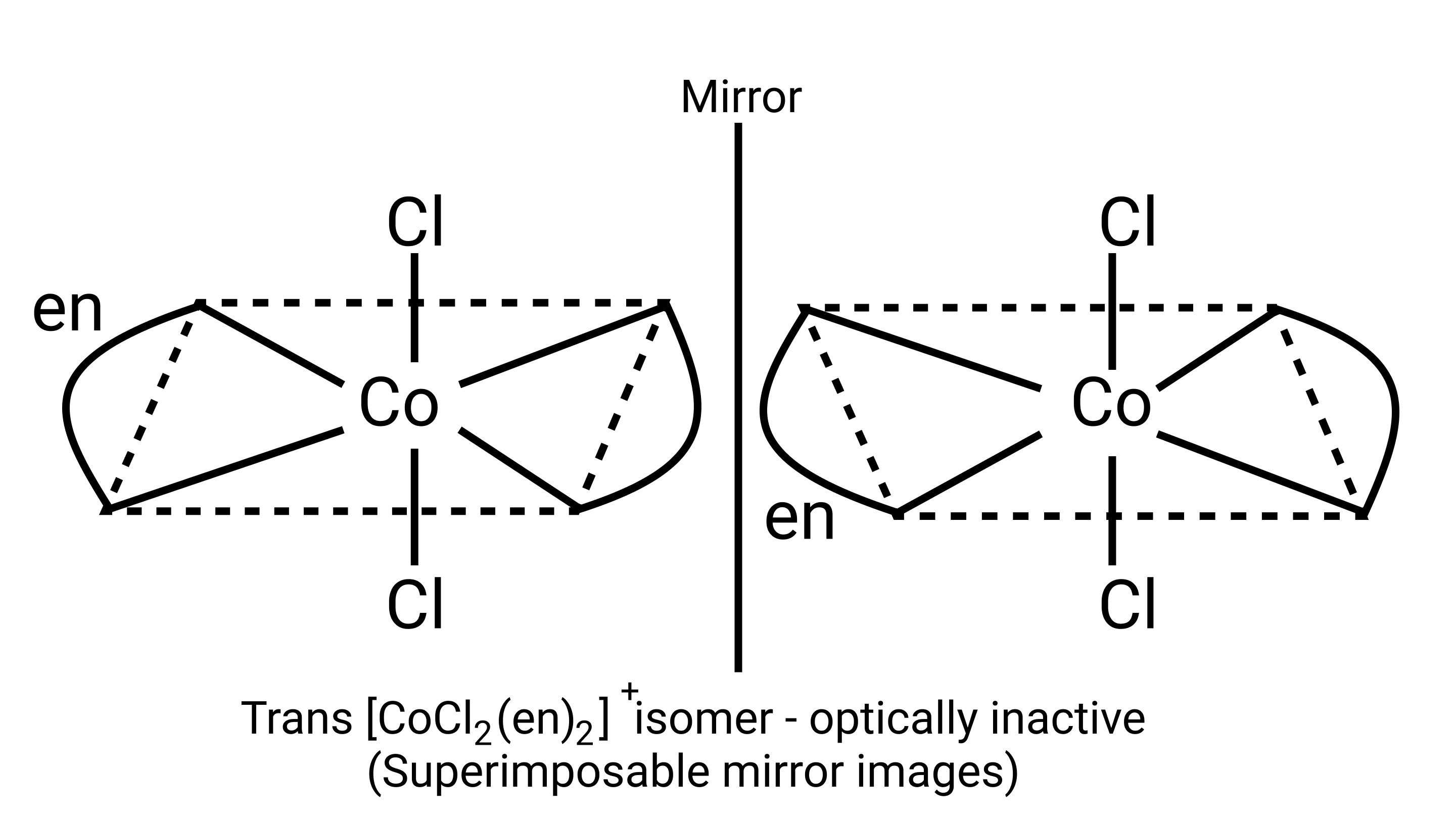
Optical compounds are highly imposable compounds that lack symmetry components. As a result, Option A and C are mirror reflections of each other.
22. Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
A: It is a neutral ligand.
B: It is a didentate ligand.
C: It is a chelating ligand.
D: It is a unidentate ligand.
Ans: Correct options: A, B and C
Option A, B, and C describe that ethane-1, 2-diamine is a neutral ligand due to its lack of charge, a bidentate ligand due to the presence of two donor sites on one nitrogen atom, and a chelating ligand due to its ability to chelate with metal.
23. Which of the following complexes show linkage isomerism?
A: ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{(N}}{{\text{O}}_{\text{2}}}{\text{)}}} \right]^{{\text{2 + }}}}$
B: ${\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{5}}}} \right]^{{\text{3 + }}}}$
C: ${\left[ {{\text{Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{SCN}}} \right]^{{\text{2 + }}}}$
D: ${\left[ {{\text{Fe(en}}{{\text{)}}_{\text{5}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}$
Ans: Correct option: C
Coordination compounds with the same composition but varying ligand-metal connectivity are known as linkage isomerism.
${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{(N}}{{\text{O}}_{\text{2}}}{\text{)}}} \right]^{{\text{2 + }}}}$, ${\text{N}}{{\text{O}}_{\text{2}}}$ has two donor sites N and O
${\left[ {{\text{Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{SCN}}} \right]^{{\text{2 + }}}}$, S and N are two donor sites.
III. Short Answer Type
24. Arrange the following complexes in the increasing order of conductivity of their solution: $\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{3}}}} \right]{\text{, }}\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{Cl, }}\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}{\text{ , }}\left[ {{\text{Cr}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{5}}}{\text{Cl}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$
Ans: Complexes with more number of ions show more conductivity.
$\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{3}}}{\text{C}}{{\text{l}}_{\text{3}}}} \right]{\text{, }}\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{ < }}\left[ {{\text{Cr}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{5}}}{\text{Cl}}} \right]{\text{C}}{{\text{l}}_{\text{2}}} < {\text{Cl, }}\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{3}}}{\text{ , }}$
25. A coordination compound ${\text{CrC}}{{\text{l}}_{\text{3}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
Ans: The solution ${\text{CrC}}{{\text{l}}_{\text{3}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ molar conductance shows that it contains one positive and one negative ion.
An ion is present outside the complex when silver chloride is treated with silver nitrate chloride. Outside the complex, there are two ions and one chloride, hence the name of the complex will be
$\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{Cl}}$, Tetraaquadichloridocobalt(III)chloride.
26. A complex of the type ${\left[ {{\text{M(AA}}{{\text{)}}_{\text{2}}}{{\text{X}}_{\text{2}}}} \right]^{{\text{n + }}}}$ is known to be optically active. What does this indicate about the structure of the complex? Give one example of such a complex.
Ans: ${\left[ {{\text{M(AA}}{{\text{)}}_{\text{2}}}{{\text{X}}_{\text{2}}}} \right]^{{\text{n + }}}}$ is a bidentate ligand and %${\text{X}}$ is a monodentate ligand. It is an example of octahedral geometry as well as an ion. There are cis and trans isomers of this ion. Trans isomers are symmetrically active, while cis isomers are optically active.
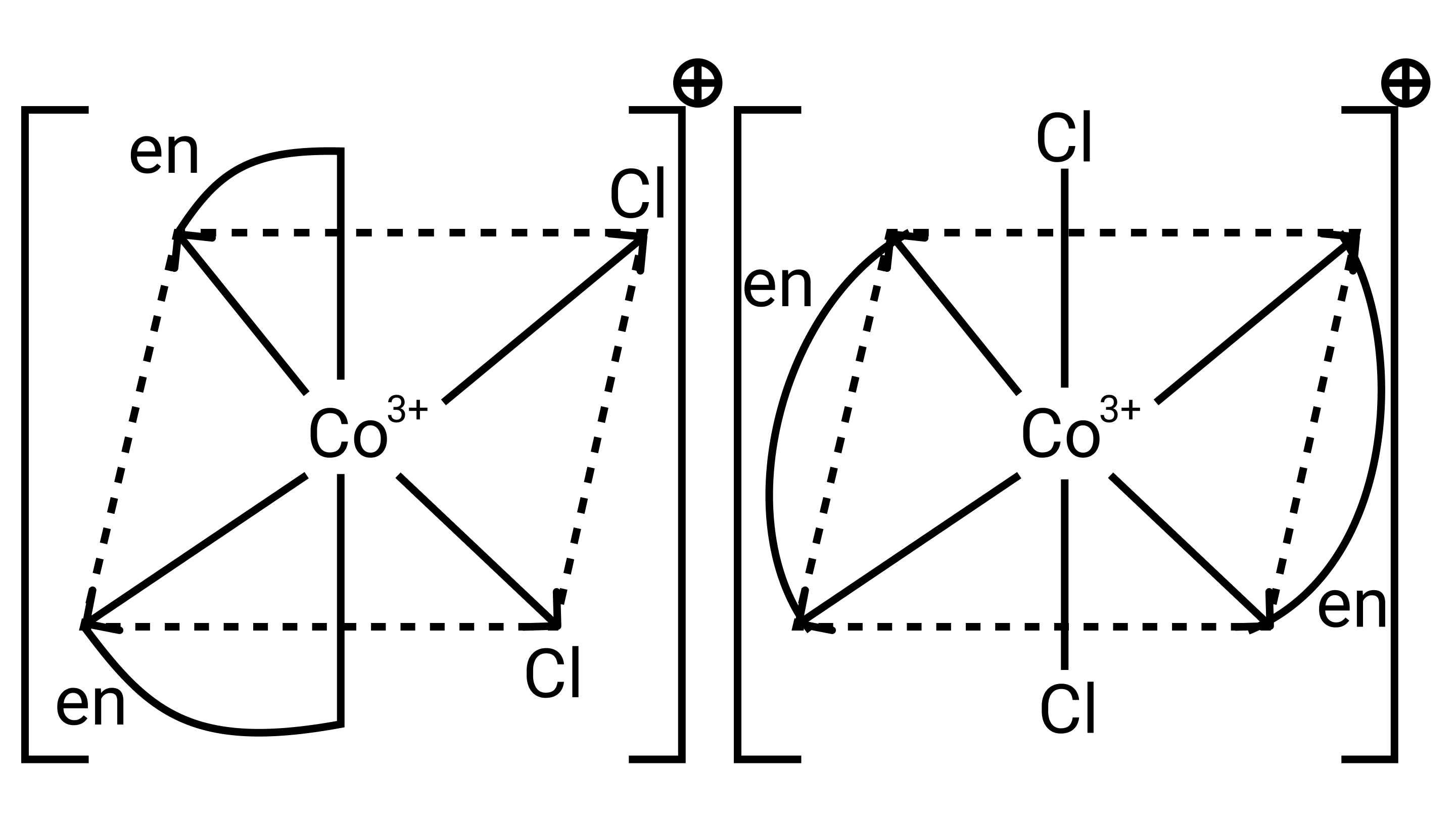
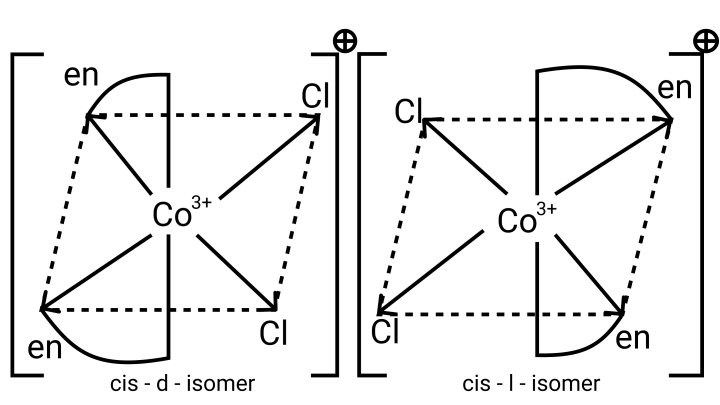
27. Magnetic moment of ${\left[ {{\text{MnC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}$ is 5.92 BM. Explain giving reason.
Ans: The existence of five unpaired electrons in the ion's mid-orbits ${\text{M}}{{\text{n}}^{{\text{2 + }}}}$ equates to a magnetic moment of 5.92 BM. The number of hybridizations in this case is ${\text{s}}{{\text{p}}^{\text{3}}}$. As a result, the magnetic moment value of the tetrahedral structure complex is 5.92 BM.
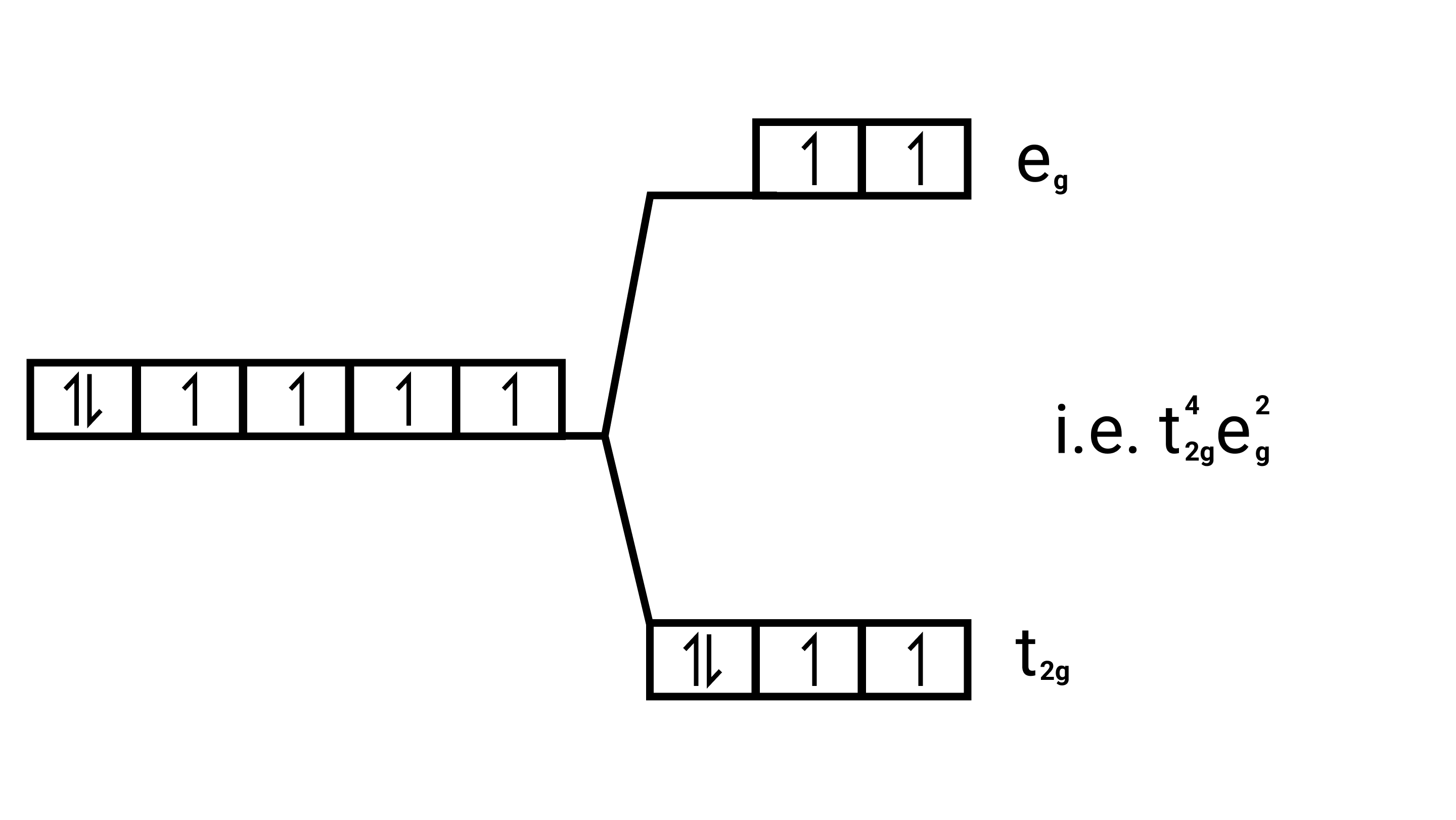
28. Based on crystal field theory explain why ${\text{Co(III)}}$ forms a paramagnetic octahedral complex with weak field ligands whereas it forms a diamagnetic octahedral complex with strong field ligands.
Ans: Weak field ligands, Electronic configuration of ${\text{Co(III)}}$ is ${{\text{t}}^{\text{4}}}_{{\text{2g}}}{{\text{e}}^{\text{0}}}_{\text{g}}$, has 4 paired electrons and it is paramagnetic.
Strong field ligands, Electronic configuration of is ${\text{Co(III)}}$ ${{\text{t}}^{\text{6}}}_{{\text{2g}}}{{\text{e}}^{\text{0}}}_{\text{g}}$.
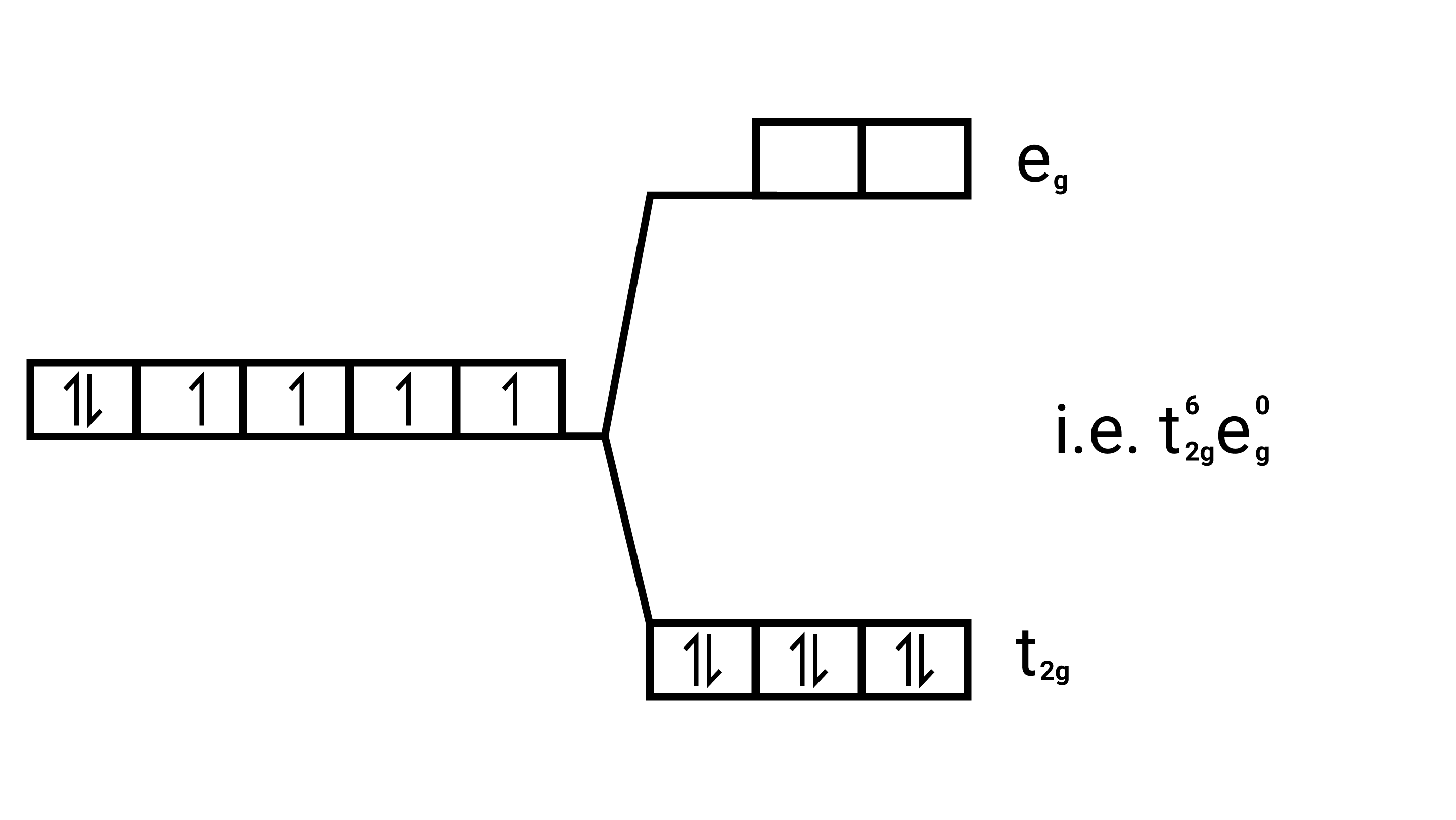
29. Why are low spin tetrahedral complexes not formed?
Ans: In a tetrahedral complex, the d-orbit splits too little compared to an octahedral complex. As a result, orbital energy alone are insufficient to couple. Low spin tetrahedral complexes do not form as a result.
30. Give the electronic configuration of the following complexes based on Crystal Field Splitting theory ${\left[ {{\text{Co}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{,}}{\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{4 - }}}}{\text{and}}{\left[ {{\text{Cu(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}$
Ans: The ligands can be grouped in ascending order of increasing field intensity according to spectrochemical series.
${{\left[ Co{{F}_{6}} \right]}^{\ 3-}},C{{o}^{3+\text{ }}}\to ({{d}_{6}})\to {{t}^{4}}_{2g}{{e}^{2}}_{g}$
\[[Fe(CN)_{6}]^{4-}, Fe^{2+} \rightarrow (d_{6}) \rightarrow t^{6}_{2g} e^{2}_{g}\]
\[[Cu(NH_{3})_{6}]^{2+}, Cu^{2+} \rightarrow (d_{9}) \rightarrow t^{6}_{2g} e^{3}_{g}\]
31. Explain why ${\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$ has magnetic moment value of 5.92 BM whereas ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$ has a value of only 1.74 BM.
Ans: ${\text{C}}{{\text{N}}^{\text{ - }}}$ is a strong ligand and ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$ has one paired electron
${{\text{H}}_{\text{2}}}{\text{O}}$ is a weak ligand and ${\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$ has five paired electrons.
32. Arrange following complex ions in increasing order of crystal field splitting energy (${\Delta{ o}}$)
${\left[ {{\text{Cr(Cl}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{,}}{\left[ {{\text{Cr(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{,}}{\left[ {{\text{Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$
Ans: CFSE is higher when the complex contains strong field ligand. Thus, crystal field splitting energy increases in the order
$\left[\mathrm{Cr}(\mathrm{Cl})_{6}\right]^{3-}<\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}<\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-} .$
Because according to spectrochemical series the order of field strength is
$C l^{-}<N H_{3}<C N^{-}$
33. Why do compounds having similar geometry have different magnetic moments?
Ans: Due to the presence of weak and strong field ligands in complexes, compounds with comparable geometry have distinct magnetic moments. The magnetic moment decreases when the CFSE increases, and vice versa.
Example: ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{ - F}}{{\text{e}}^{{\text{3 + }}}}{\text{,3}}{{\text{d}}^{\text{5}}}{\text{,C}}{{\text{N}}^{\text{ - }}}$(strong field ligand, pairs electron)
${\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{ - F}}{{\text{e}}^{{\text{3 + }}}}{\text{,3}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{H}}_{\text{2}}}{\text{O}}$ (weak field ligand, does not pair)
34. ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$ is blue in colour while ${\text{CuS}}{{\text{O}}_{\text{4}}}$ is colourless. Why?
Ans: In $\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}$, water acts as ligand and causes crystal field splitting. This makes d - d transitions possible. On the other hand, in $\mathrm{CuSO}_{4}$, splitting is not possible due to the lack of ligand in the crystal field. As a result, no colour is visible.
35. Name the type of isomerism when ambidentate ligands are attached to central metal ion. Give two examples of ambidentate ligands.
Ans: Ambidentate ligands are ligands which have two donating sites. Coordinating compounds containing ambidentate ligands show linkage isomerism due to two different binding positions. Linkage isomerism have same ligand and geometry attached to a central metal ion by different donating sites
Examples:
(i)
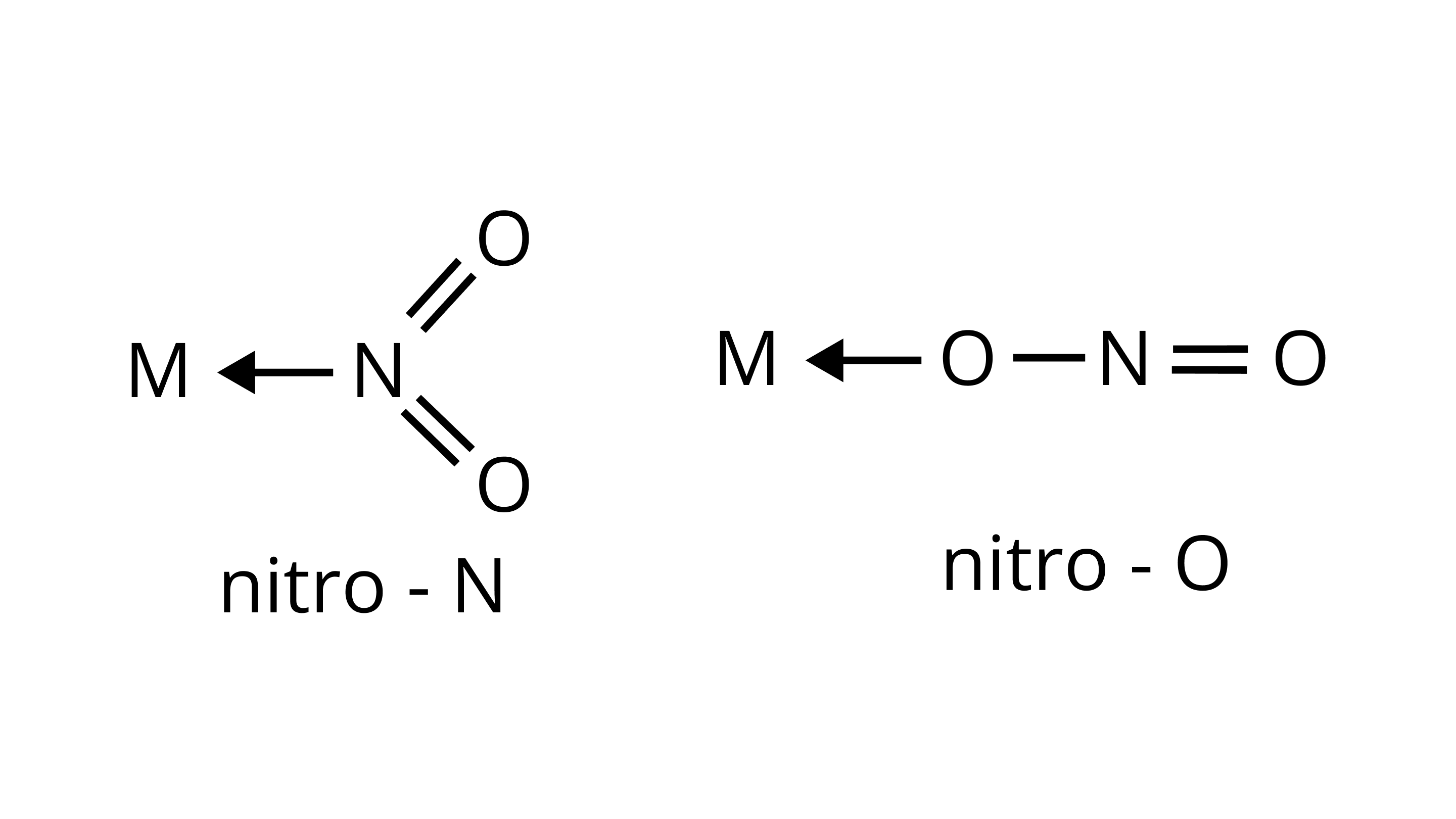
(ii) $\mathrm{M} \leftarrow \mathrm{SCN}$
thiocyanato
$\mathrm{M} \leftarrow \mathrm{NCS}$
isothiocyanato
IV. Matching Type
Note: In the following questions match the items given in Columns I and II
36. Match the complex ions given in Column I with the colours given in Column II and assign the correct code
Column I (Complex ion) | Column II (Colour) |
A. ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$ | 1. Violet |
B. ${\left[ {{\text{Ti(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$ | 2. Green |
C. ${\left[ {{\text{Ni(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}$ | 3. Pale blue |
D. ${\left[ {{\text{Ni(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{4}}}{\text{(en}}} \right]^{{\text{2 + }}}}{\text{(aq)}}$ | 4. Yellowish orange |
5. Blue |
Code
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Ans: Correct option: (ii)
37. Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code
Column I (Coordination Compound) | Column II (Central metal atom) |
A. Chlorophyll | 1. Rhodium |
B. Blood pigment | 2. Cobalt |
C. Wilkinson catalyst | 3. calcium |
D. Vitamin B12 | 4. iron |
5. magnesium |
Code
(i) A (5) B (4) C (1) D (2)
(ii) A (3) B (4) C (5) D (1)
(iii) A (4) B (3) C (2) D (1)
(iv) A (3) B (4) C (1) D (2)
Ans: Correct option: (i)
38. Match the complex ions given in Column I with the hybridisation and number of unpaired electrons given in Column II and assign the correct code
Column I (Complex ion) | Column II (Hybrisization, number of unpaired electrons) |
A. ${\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}$ | 1. ${\text{ds}}{{\text{p}}^{\text{2}}}{\text{,1}}$ |
B. ${\left[ {{\text{Co(CN}}{{\text{)}}_{\text{4}}}} \right]^{{\text{2 - }}}}$ | 2. ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}{\text{,5}}$ |
C. ${\left[ {{\text{Ni(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}$ | 3. ${{\text{d}}^{\text{2}}}{\text{s}}{{\text{p}}^{\text{3}}}{\text{,3}}$ |
D. ${\left[ {{\text{Mn}}{{\text{F}}_{\text{6}}}} \right]^{{\text{4 - }}}}$ | 4. ${\text{s}}{{\text{p}}^{\text{3}}}{\text{,4}}$ |
5. ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}{\text{,2}}$ |
Code
(i) A (3) B (1) C (5) D (2)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Ans: Correct option: (ii)
39. Match the complex species given in Column I with the possible isomerism given in Column II and assign the correct code
Column I (Complex species) | Column II (Isomerism) |
A. ${\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}$ | 1. Optical |
B. ${\text{cis - }}{\left[ {{\text{Co(en}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^{\text{ + }}}$ | 2. Ionisation |
C. $\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{(N}}{{\text{O}}_{\text{2}}}{\text{)}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ | 3. Coordination |
D. $\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]$ | 4. geometrical |
5. linkage |
Code :
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (4) B (1) C (5) D (3)
(iv) A (4) B (1) C (2) D (3)
Ans: Correct option: (iv)
40. Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code.
Column I (Compound) | Column II (Oxidation state of ${\text{Co}}$) |
A. $\left[ {{\text{Co(NCS)(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{(S}}{{\text{O}}_{\text{3}}}{\text{)}}} \right]$ | 1. + 4 |
B. $\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{4C}}{{\text{l}}_{\text{2}}}} \right]{\text{S}}{{\text{O}}_{\text{4}}}$ | 2. 0 |
C. ${\text{N}}{{\text{a}}_{\text{4}}}\left[ {{\text{Co(}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{3}}}} \right]$ | 3. +1 |
D. $\left[ {{\text{C}}{{\text{o}}_{\text{2}}}{{{\text{(CO)}}}_{\text{8}}}} \right]$ | 4. +2 |
5. +3 |
Code
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (5) B (1) C (4) D (2)
(iv) A (4) B (1) C (2) D (3)
Ans: Correct option: (i)
V. Assertion and Reason Type
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
A: Assertion and reason both are true, reason is correct of assertion.
B: Assertion and reason both are true, but reason is not the correct of assertion.
C: Assertion is true, reason is false.
D: Assertion is false, reason is true
41. Assertion: Toxic metal ions are removed by the chelating ligands.
Reason: Chelate complexes tend to be more stable.
Ans: Correct option: A
Toxic metal ions are removed by the chelating ligands. Chelate complexes tend to be more stable. When a solution of the chelating ligand is added to a solution containing toxic metals ligands chelates the metal ions by the formation of a stable complex.
Thus, EDT A (hexadentate ligand) forms a stable metal chelate and is used to remove toxic metal ions.
Both Assertion and Reason are correct and Reason is the correct for Assertion.
Hence, option A is correct.
42. Assertion : $\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ and $\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ are reducing in nature.
Reason: Unpaired electrons are present in their d-orbitals.
Ans: Correct option: B
$\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ and $\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$
Due to the creation of a more stable complex ion after obtaining ion, $\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ and $\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$ are reduced in nature. Both compounds have a coordination of 6 and so form an octahedral complex. The hybridisation state of is 3 and the oxidation state of is 2+. Hybridization is ${\text{3}}{{\text{d}}^{\text{4}}}$ and ${\text{3}}{{\text{d}}^{\text{6}}}$ is 2+. Both compounds have unpaired electrons and have weak field ligands. In both compounds, removing electrons is simple.
43. Assertion: Linkage isomerism arises in coordination compounds containing ambidentate ligand.
Reason: Ambidentate ligand has two different donor atoms.
Ans: Correct option: A
The term "linkage isomerism" refers to two molecules that have similar ligands but differ in the donating site of the ligand. Ambidentate is made up of two different types of donor atoms.
Example $\left[ {{\text{Fe(N}}{{\text{O}}_{\text{2}}}{\text{)(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{5}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$. ${\text{N}}{{\text{O}}_{\text{2}}}$ is Ambidentate ligand as ${\text{N}}$and ${{\text{O}}_{\text{2}}}$ are donors.
As a result, linkage isomerism occurs in coordinations with ambidentate ligands. Reason and Assertion are both correct.
44. Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Ans: Correct option: B
From the Cis and Trans forms, geometrical isomerism forms compounds. All of the valences in the MX6 ligand are the same, resulting in an identical molecule. L is a distinct ligand in MX5L, and it can be replaced with any other valency in the structure to create an identical molecule; the molecule does not change. Because of the presence of symmetry, which is an essential prerequisite for revealing geometrical isomerism, complexes do not show geometrical isomerism.
45. Assertion: ${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$ ion shows magnetic moment corresponding to two unpaired electrons.
Reason: Because it has d ${\text{2s}}{{\text{p}}^{\text{3}}}$ type hybridisation.
Ans: Correct option: D
${\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$ ion shows magnetic moment corresponding to one unpaired electron. $\left( {{\text{CN}}} \right)$ is a ligand with a strong field that couples electrons, resulting in hybridisation. ${{\text{d}}^{\text{2}}}{\text{s}}{{\text{p}}^{\text{3}}}$ and it has one unpaired electron, it has the magnetic moment of one paired electron.
$\mu = \sqrt {{\text{n(n + 2)}}}$
${\text{ = }}\sqrt {{\text{1(1 + 2)}}}$
${\text{ = }}\sqrt {\text{3}} {\text{ = 1}}{\text{.73BM}}$
VI. Long Answer Type
46. Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following:
(i) ${\left[ {{\text{Co}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{,}}{\left[ {{\text{Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}{\text{,}}{\left[ {{\text{Co(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}$
Ans: Electronic cnfiguration: $\mathrm{Co}^{3+}=[\mathrm{Ar}] 3 \mathrm{~d}^{6}$
Energy level diagram:
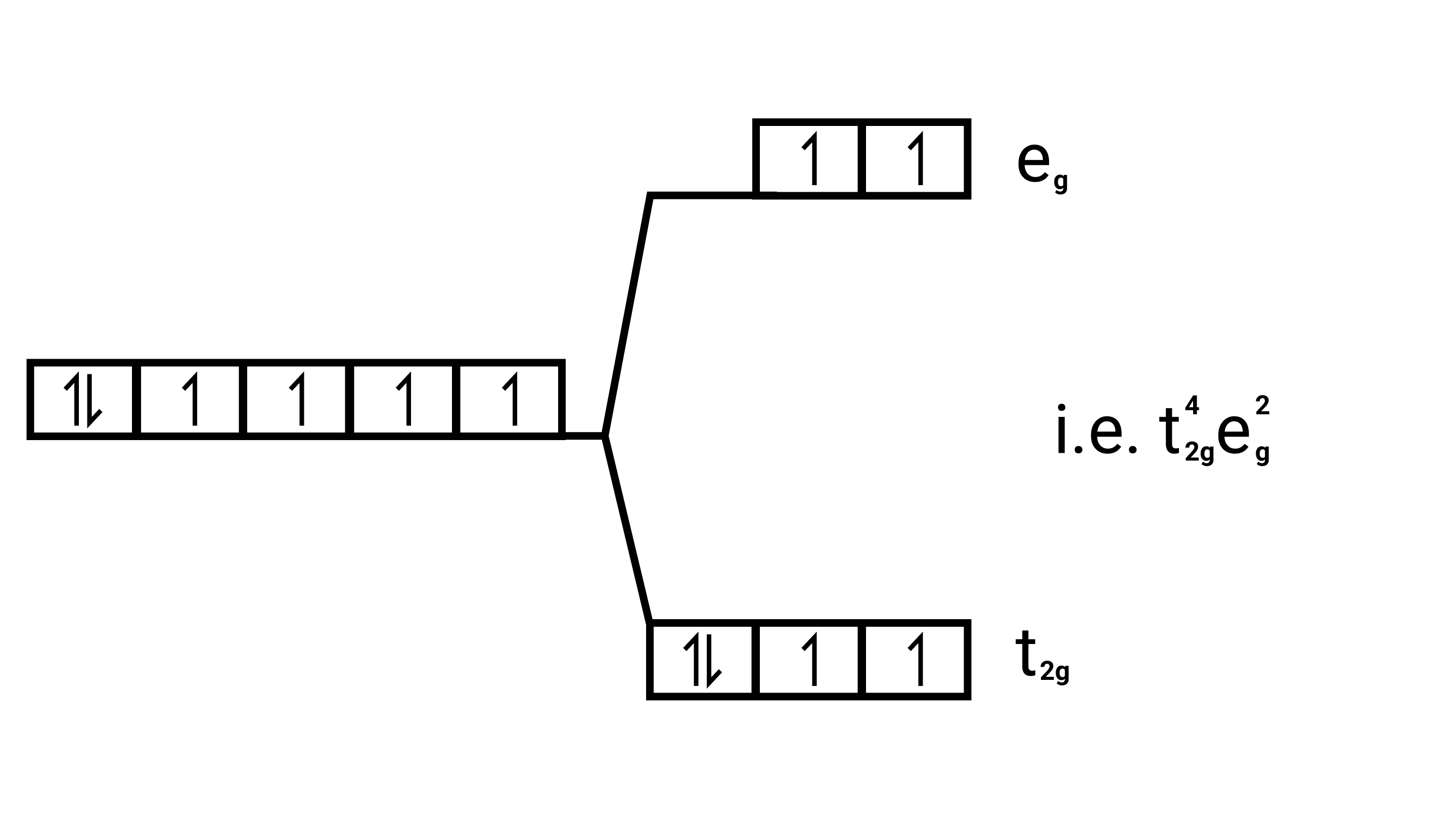
Magnetic moment:
Number of unpaired electrons $(n)=4$
Magnetic moment $=\sqrt{n(n+2)}=\sqrt{4(4+2)}=\sqrt{24}=4.9 \mathrm{BM}$
$\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$
Electronic cnfiguration: $\mathrm{Co}^{2+}=[\mathrm{Ar}] 3 \mathrm{~d}^{7}$
Energy level diagram:
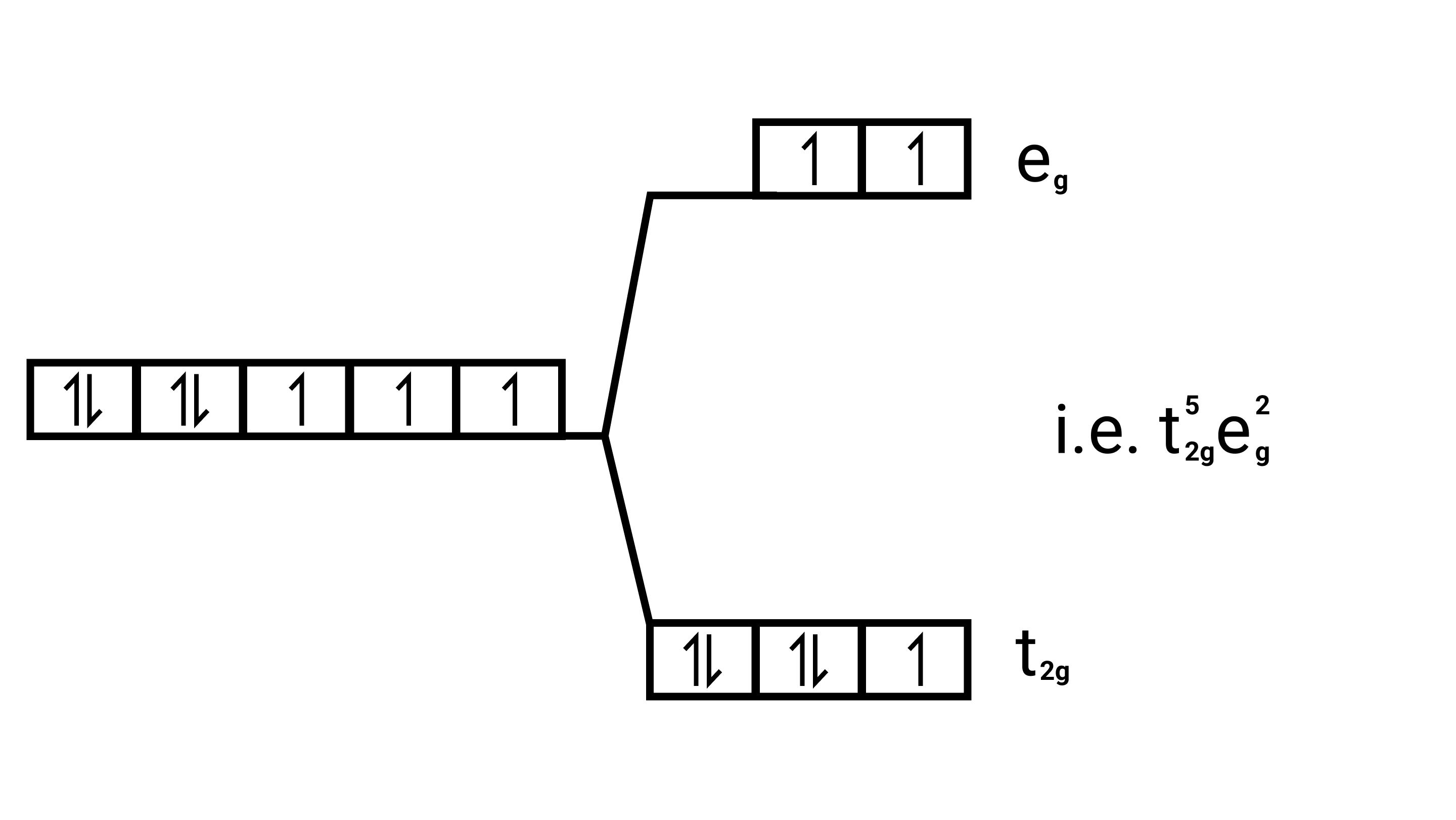
Magnetic moment: Since ,number of unpaired electrons $(\mathrm{n})=3$, therefore magnetic moment $=\sqrt{3(3+2)}=\sqrt{15}=3.87 \mathrm{BM}$
$\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}$
Electronic configuration: $[\mathrm{Ar}] \mathrm{Co}^{3+}=3 \mathrm{~d}^{6}$
Energy level diagram:
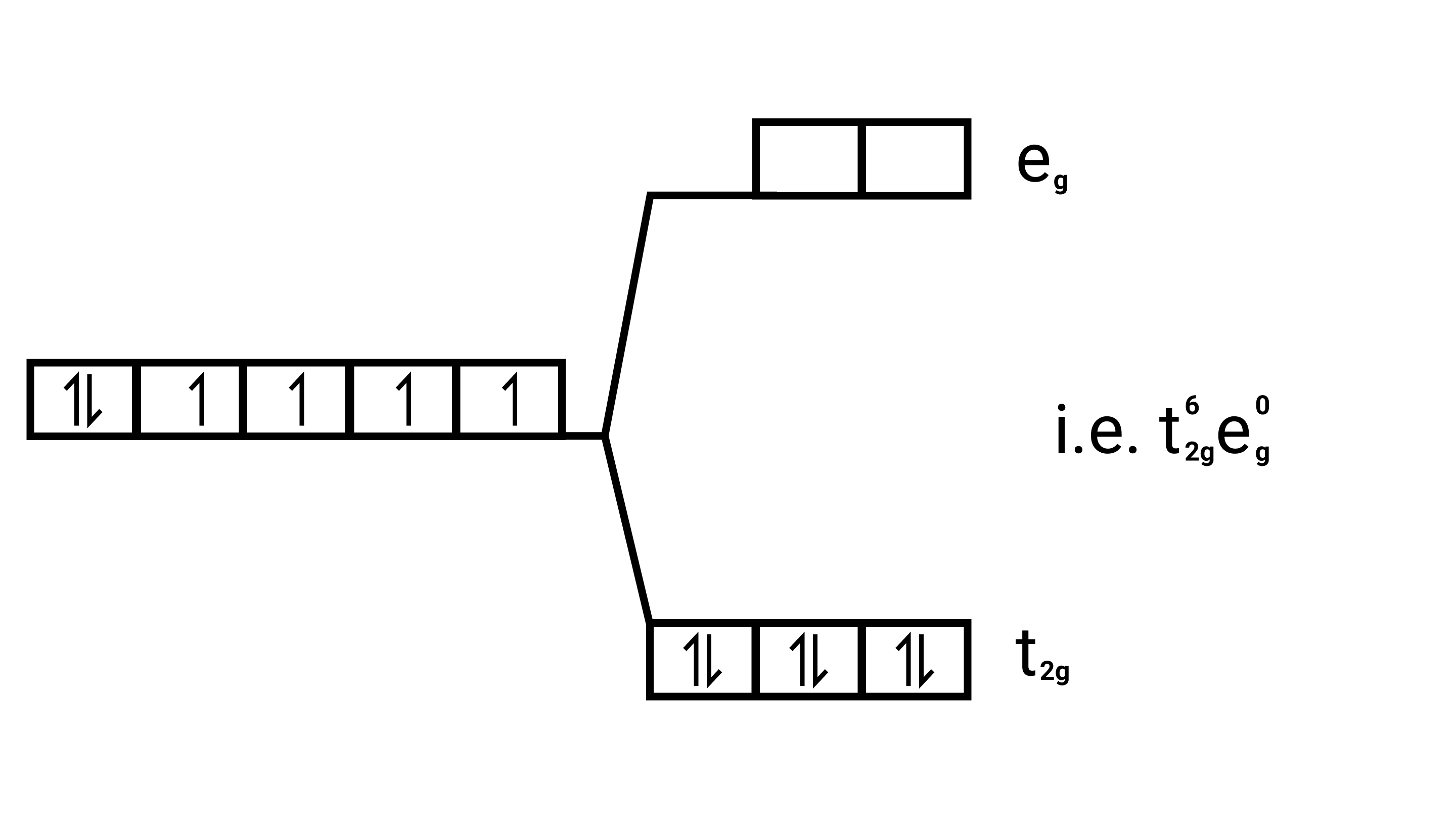
(ii) ${\left[ {{\text{Fe}}{{\text{F}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{,}}{\left[ {{\text{Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{2 + }}}}{\text{,}}{\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{4 - }}}}$
Ans: $\left[\mathrm{FeF}_{6}\right]^{3-}$
Electronic configuration: $\mathrm{Fe}^{3+}=[\mathrm{Ar}] 3 \mathrm{~d}^{5}$
Energy level diagram:
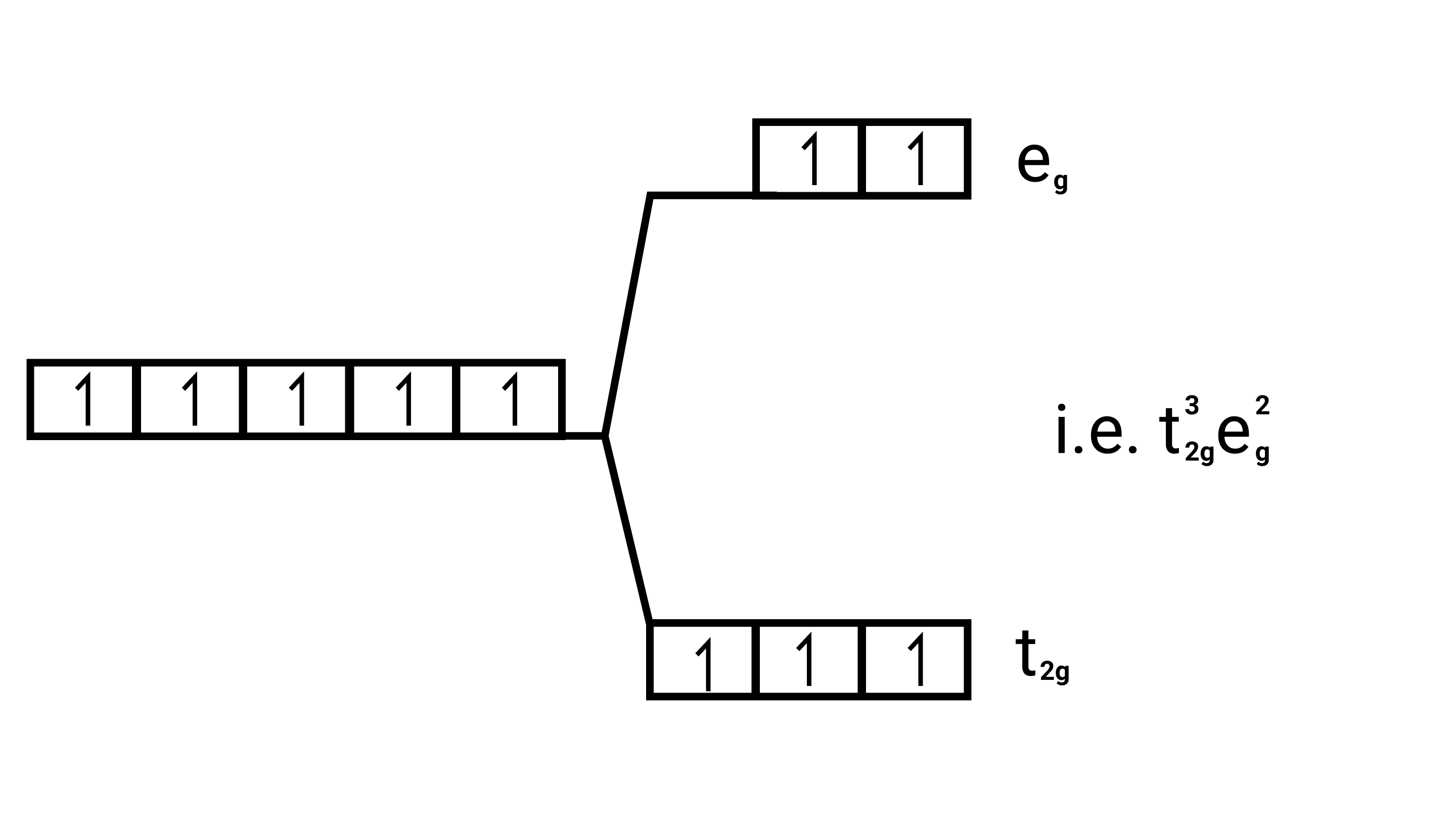
Number of unpaired electrons $=5$ $\sqrt{5(5+2)}=\sqrt{35}=5.95 \mathrm{BM}$ $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$
$\mathrm{Fe}^{2+}=[\mathrm{Ar}] 3 \mathrm{~d}^{6}$
Energy level diagram:
Magnetic moment
Since, number of unpaired electrons $(\mathrm{n})=4$, therefore magnetic moment is-
$\sqrt{4(4+2)}=\sqrt{24}=4.9 \mathrm{BM}$
$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}$
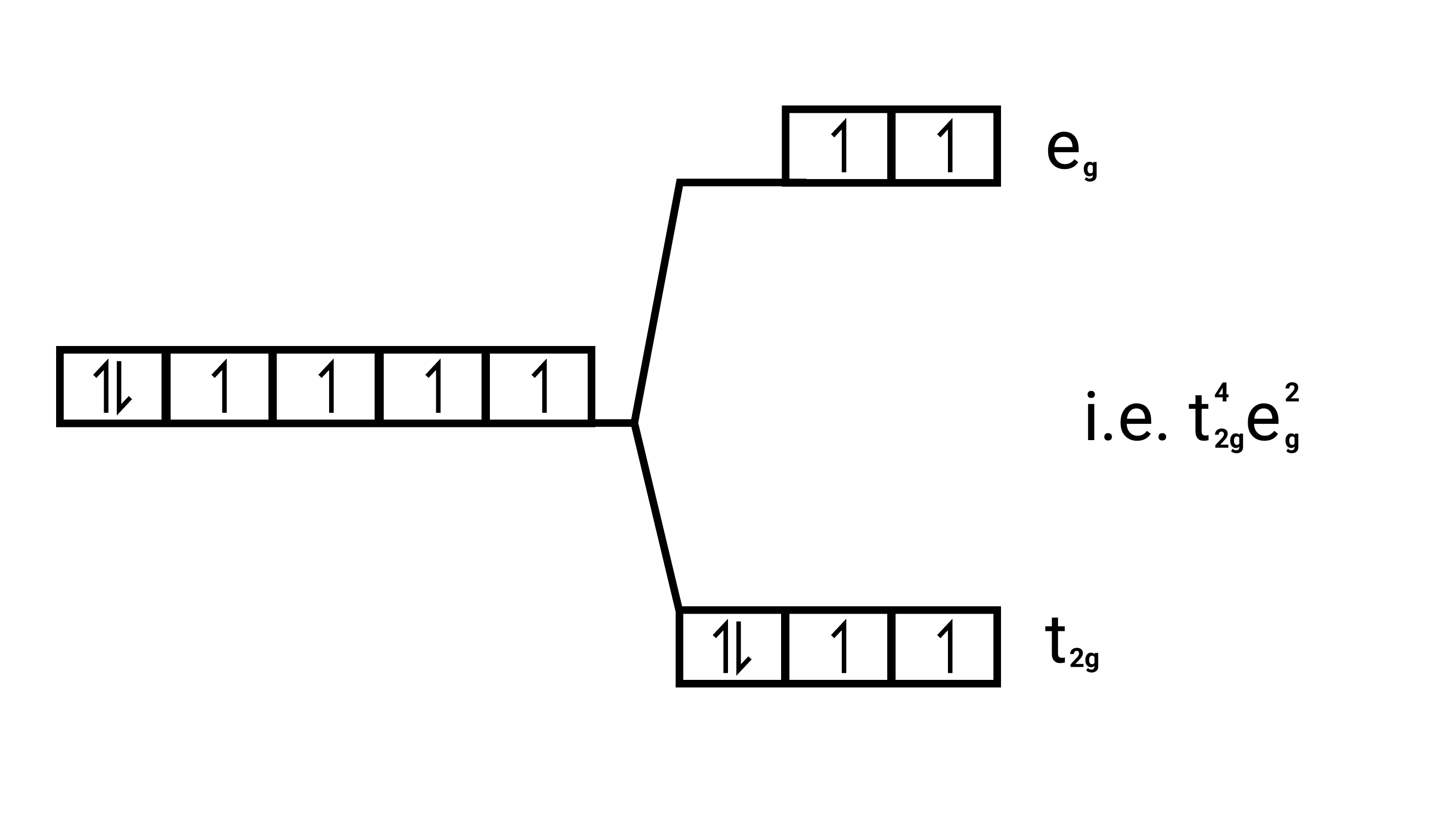
Electronic configuration: $\mathrm{Fe}^{2+}=[\mathrm{Ar}] 3 \mathrm{~d}^{6}$
Energy level diagram
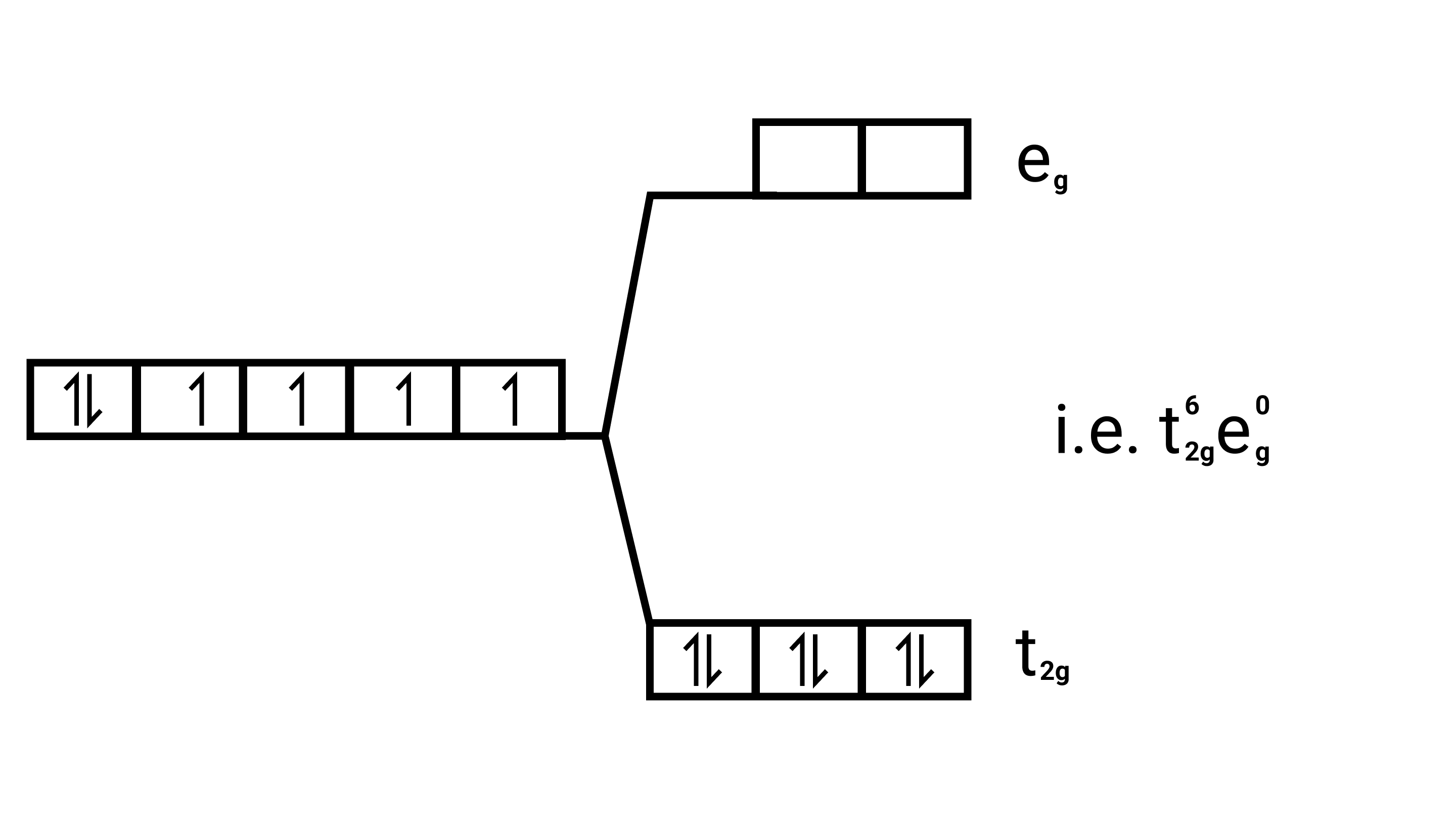
Since $\mathrm{CN}^{-}$is strong field ligand, it forces all the electrons to get paired up Magnetic moment.
In the absence of unpaired electrons it behaves as diamagnetic ( ie. no magnetic behaviour ).
So magnetic moment $=$ zero
47. Using valence bond theory, explain the following in relation to the complexes given below:
${\left[ {{\text{Mn(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}{\text{,}}{\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}{\text{,}}{\left[ {{\text{Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}{\text{,}}{\left[ {{\text{FeCl}}} \right]^{{\text{4 - }}}}$
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value
Ans: $\left[\operatorname{Mn}(\mathrm{CN})_{6}\right]^{3-}$
Electronic configuration is $\mathrm{Mn}^{3+}=[\mathrm{Ar}] 3 \mathrm{~d}^{4}$ hence box electronic structure
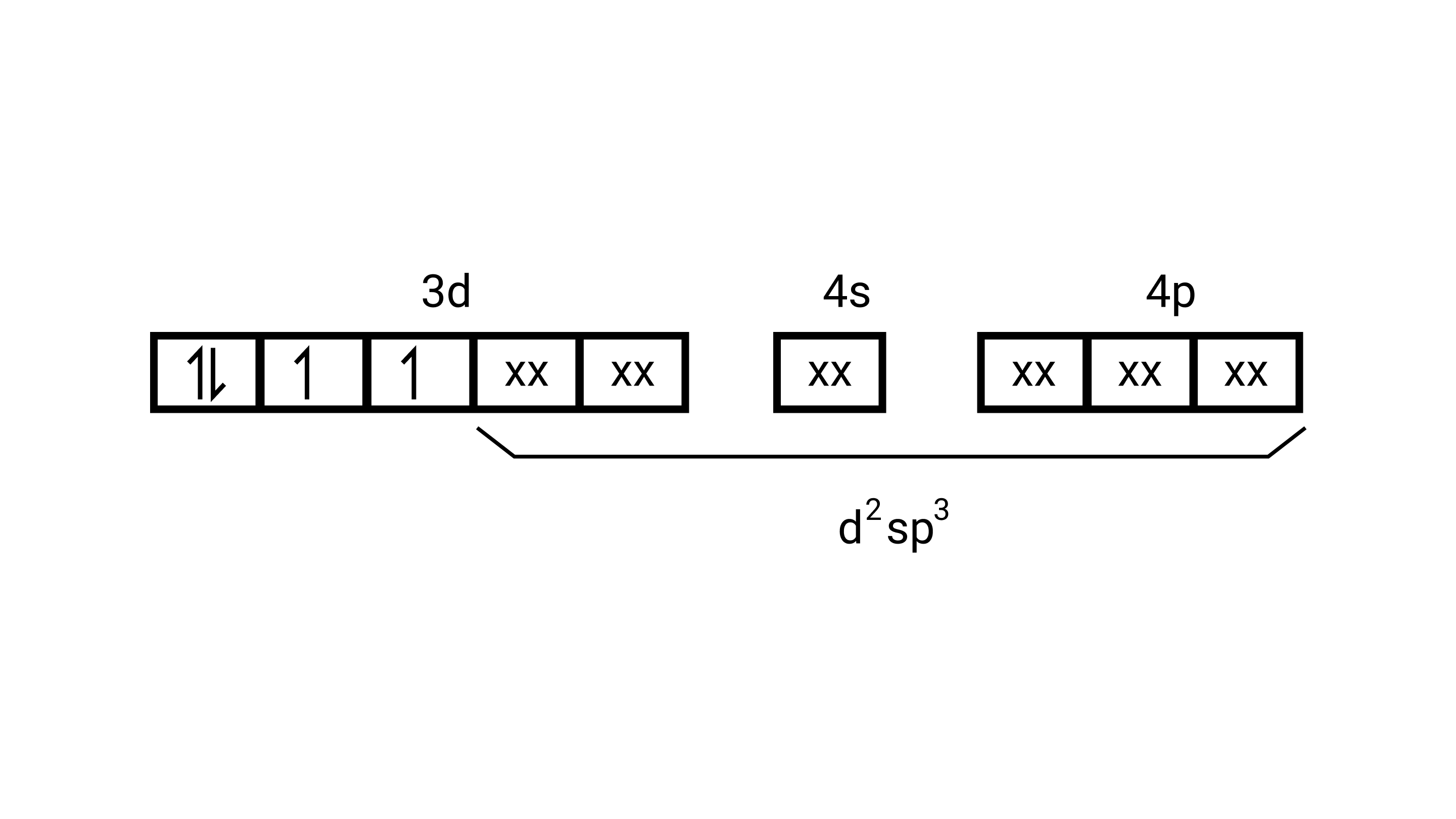
(i) Type of hybridisation $\mathrm{d}^{2} \mathrm{sp}^{3}$
(ii) Inner orbital complex
(iii) paramagnetic, due to presence of three unpaired electrons.
(iv) Spin only magnetic moment is calculated using the formula : $\mathrm{n}=2$ in this case, we get spin only magnetic moment in BMas $=\sqrt{2(2+2)}=\sqrt{8}=$
$2.87 \mathrm{BM}$
$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$
Electronic configuration of $\mathrm{Co}^{3+}=[\mathrm{Ar}] 3 \mathrm{~d}^{6}$
(i) Hyb As shown in the above box electronic structure the type of hybridisation is ........... $\mathrm{d}^{2} \mathrm{sp}^{3}$
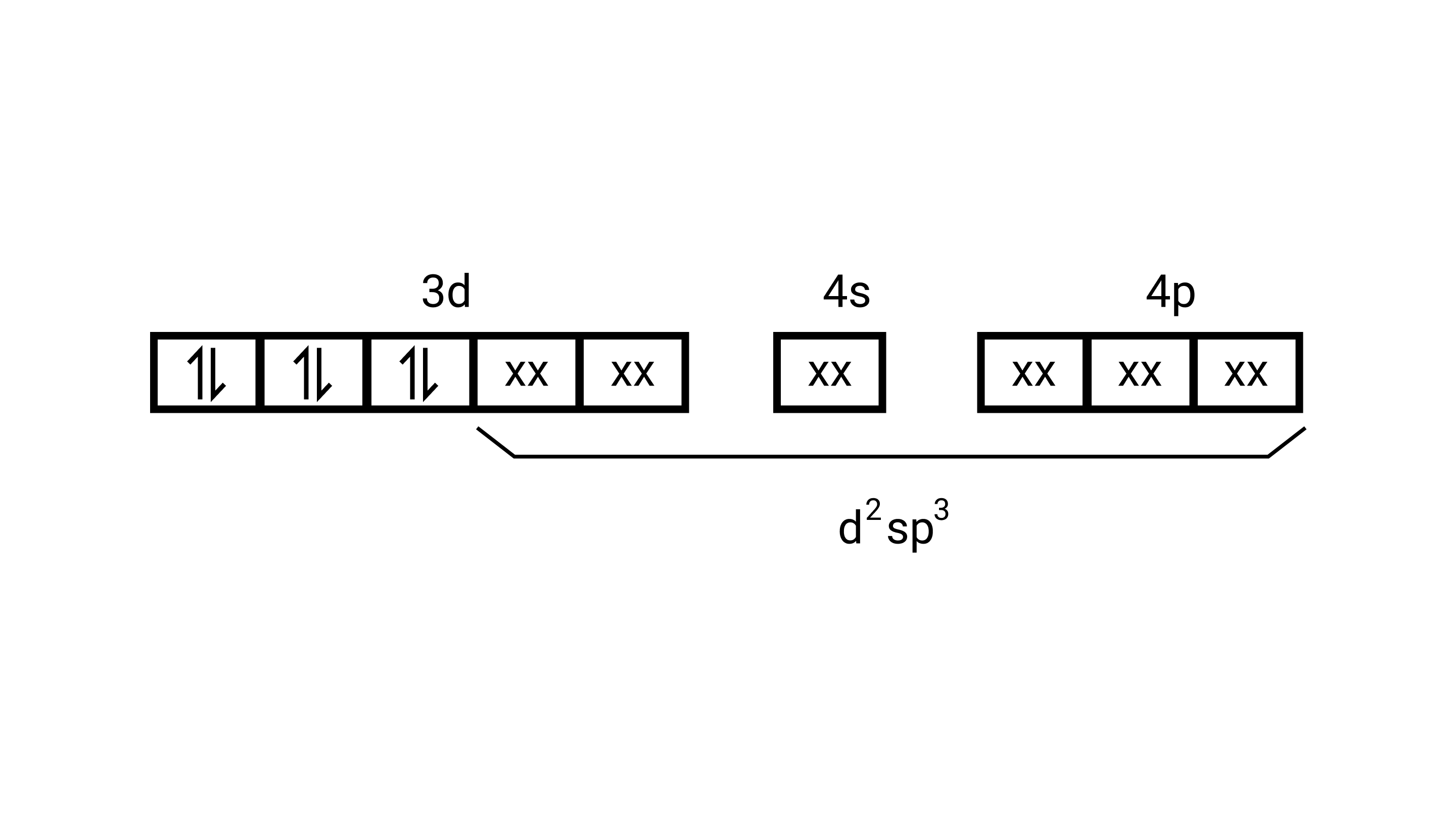
(ii) Inner orbital complex
(iii) Diamagnetic
(iv) Zero( Since no unpaired electrons are present, as depicted above)
$\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$
(i) type of hybridisation........ $\mathrm{d}^{2} \mathrm{sp}^{3}$
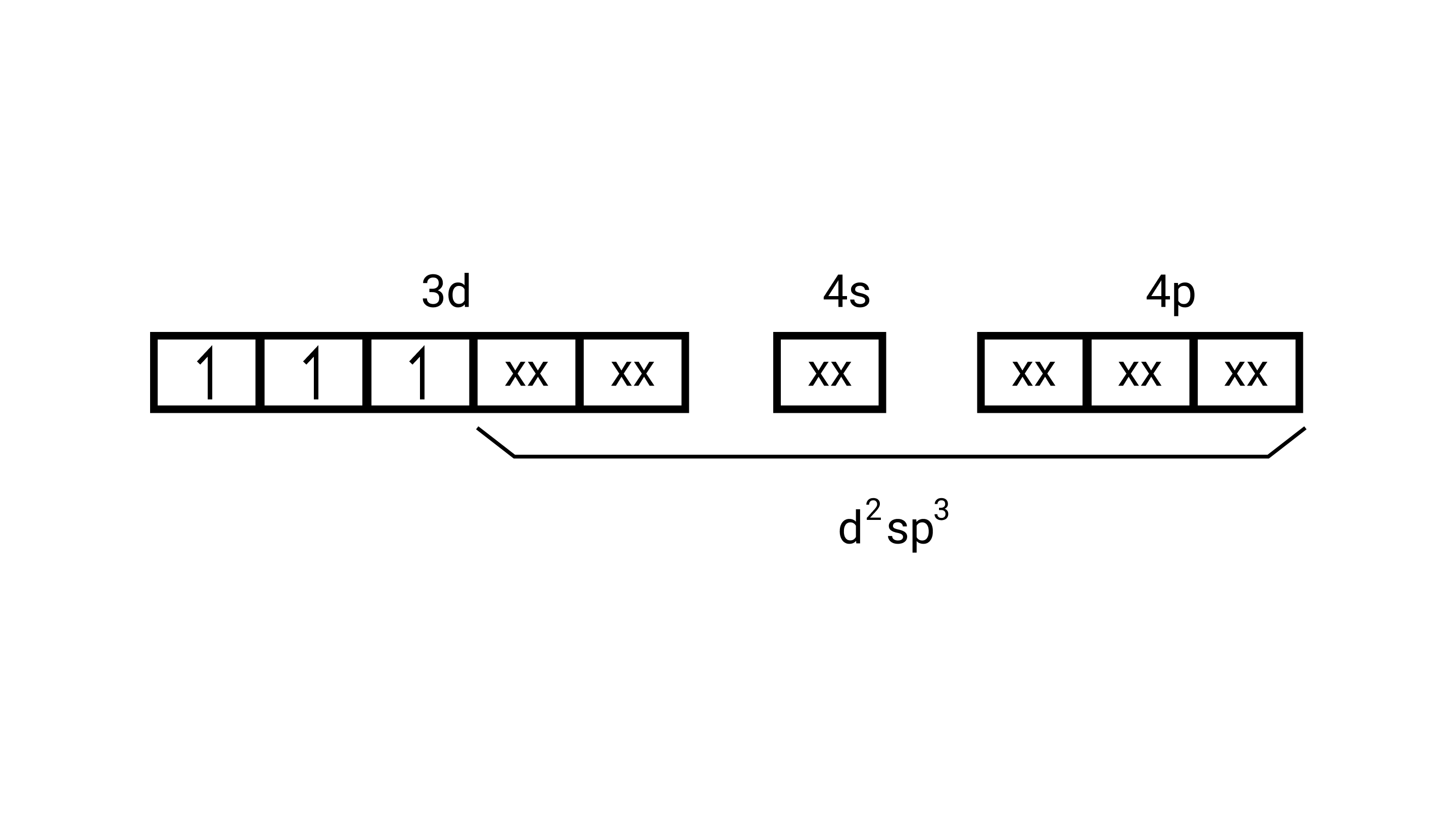
(ii) Inner orbital complex
(iii) paramagnetic
(iv) $3.87 \mathrm{BM}$
$\left[\mathrm{Fe}(\mathrm{cl})_{6}\right]^{4-}$
electronic configuration is of $\mathrm{F} \mathrm{e}^{2+}=[\mathrm{Ar}] 3 \mathrm{~d}^{6}$
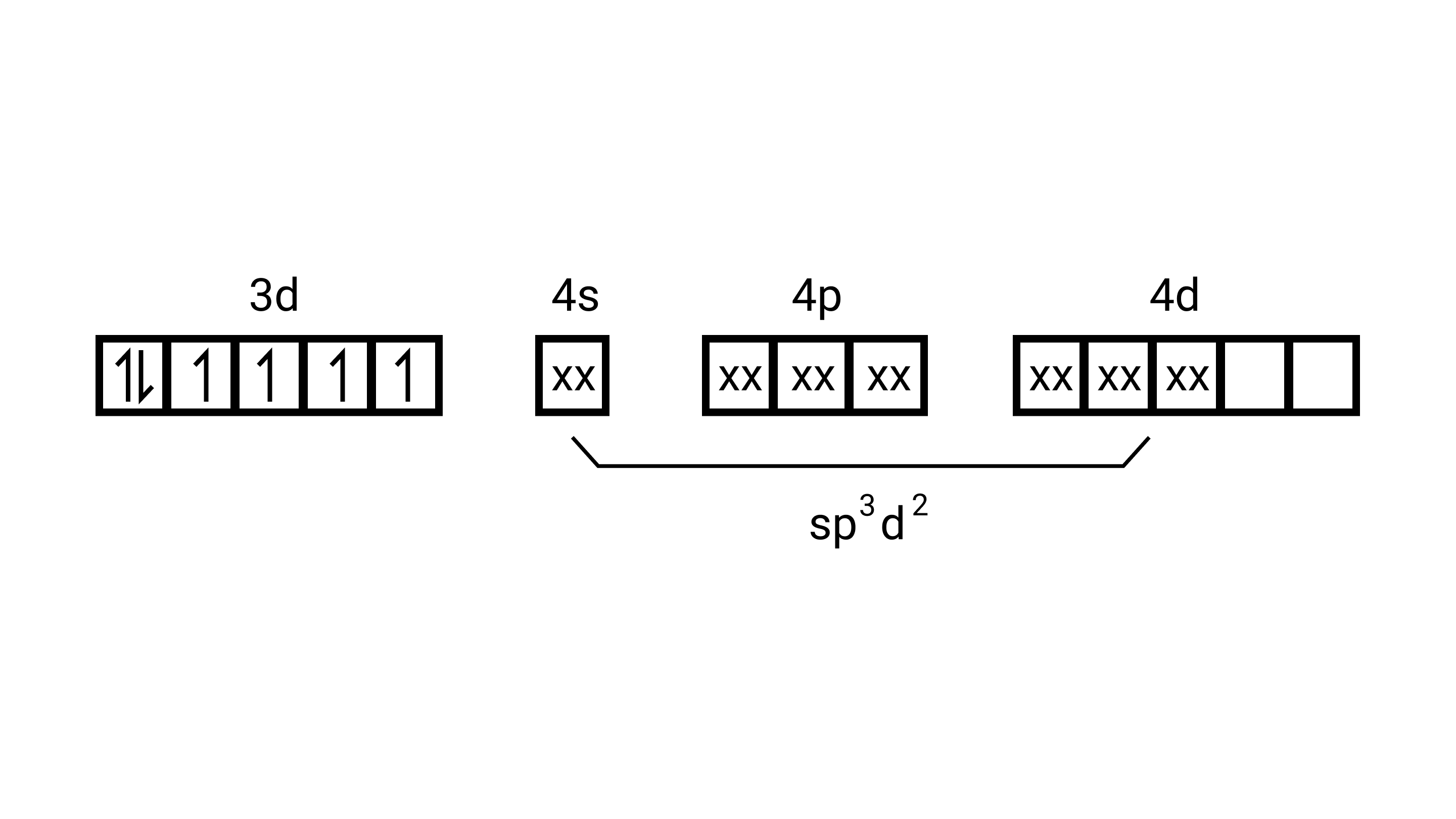
(i) type of hybridisation will be sp $^{3} \mathrm{~d}^{2}$
(ii) Outer orbital complex
(iii) Paramagnetic
(iv) $4.9 \mathrm{BM}$
48. ${\text{CoS}}{{\text{O}}_{\text{4}}}{\text{Cl}}{\text{.5N}}{{\text{H}}_{\text{3}}}$ exists in two isomeric forms ‘A’ and ‘B’. Isomer ‘A’ reacts with ${\text{AgN}}{{\text{O}}_{\text{3}}}$ to give white precipitate but does not react with ${\text{BaC}}{{\text{l}}_{\text{2}}}$ . Isomer ‘B’ gives white precipitate with ${\text{BaC}}{{\text{l}}_{\text{2}}}$ but does not react with ${\text{AgN}}{{\text{O}}_{\text{3}}}$.
Answer the following questions.
(i) Identify ‘A’ and ‘B’ and write their structural formulas.
Ans: ‘A’ is $\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{S}}{{\text{O}}_{\text{4}}}} \right]{\text{Cl}}$
‘B’ is $\left[ {{\text{Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{5}}}{\text{Cl}}} \right]{\text{S}}{{\text{O}}_{\text{4}}}$
(ii) Name the type of isomerism involved.
Ans: Type of isomerism is ionisation isomerism
(iii) Give the IUPAC name of ‘A’ and ‘B’.
Ans: IUPAC name of isomer ‘A’ is pentaaminesulphatocobalt(III)chloride
IUPAC name of isomer ‘B’ is pentaaminechlorocobalt(III)sulphate
49. What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
Ans: When light wavelengths from a specific part of the spectrum are absorbed by a substance, the result is a complimentary colour. When a complex absorbs a wavelength of light, it reflects a complementary colour. If a violent colour is absorbed, for example, yellow is conveyed. The CFSE value, often known as the colour definer for any complex, comes next. To determine the wavelength value in order to determine which colour absorbs the most energy.
${\Delta{ e = }}\frac{{{\text{hc}}}}{{\lambda{ }}}$
As ${\lambda{ }}$ has shorter wavelength.
Low spin complexes absorb shorter wavelengths, while high spin complexes absorb longer ones. The colour theory, complex, and wavelength are all determined using this method.
50. Why are different colours observed in octahedral and tetrahedral complexes for the same metal and same ligands?
Ans: The octahedral and tetrahedral splitting up of d orbitals is different.
${\Delta{ t = }}\left( {\frac{{\text{4}}}{{\text{9}}}} \right){\Delta{ o}}$
${\Delta{ t < }\Delta{ o}}$
${\Delta{ t}}$= CFSE in tetrahedral field
${\Delta{ o}}$= CFSE in octahedral field
Comparing CFSE with energy= $\frac{{{\text{hc}}}}{{\lambda{ }}}$
The lower the absorption in, the higher the CFSE. The lower wavelengths are absorbed by the octahedral complex, while the rest is transmitted. It absorbs higher wavelengths in a tetrahedral complex and transmits the rest. For the same metal and ligands, the octahedral complex has a shorter wavelength than the tetrahedral complex.
The NCERT Exemplar is a great asset that students of Class 12 can use and adapt to make sure that they have all the important answers for their upcoming test. The Exemplar has important questions that have appeared in both school-level examinations and even competitive examinations time and time again. Therefore there is no denying the fact that the NCERT Exemplar for Class 12 is a great resource that guarantees one of the most useful question resources for all tests that Class 12 students take.
The solution PDF made by Vedantu for Class 12 NCERT Exemplar that has the solutions for Coordination Compounds has been made with a lot of care and is very accurate in terms of offering the best quality of answers to students preparing for exams like JEE, NEET, CET, and even school-level exams. By using this solution PDF, students of Class 12 can:-
Solve good quality questions from Class 12 NCERT Exemplar that require a high level of thinking
Prepare for school examinations and competitive at the same time
Understand the way in which answers are written
Use the same material for group studies and mocks
Conclusion
NCERT Exemplar is a great resource and with the solutions from Vedantu, you too can take the advantage of the textbook to the fullest. The detailed and well-written solutions in the free PDF can help students score the most marks in the Chapter of Coordination Compounds.
FAQs on NCERT Exemplar for Class 12 Chemistry Chapter-9 (Book Solutions)
1. Is the Class 12 NCERT Exemplar good for NEET for the Chapter Coordination Compounds?
Coordination Compounds is a very scoring Chapter from Inorganic Chemistry for Classes 11 and 12. The two Chapters in the two Classes are very scoring since they are not very difficult and fairly easy to understand. By using the Class 12 NCERT Exemplar, students can score well in NEET, JEE, and even Class 12 Board examinations. As mentioned earlier, the level of questions in the NCERT exemplar is higher compared to the NCERT Textbook for students of Class 12, so it definitely serves its purpose of being a well-equipped resource for both competitive and school-level examinations.
2. Is it compulsory to study Coordination Compounds from Class 11 before studying it in Class 12?
Inorganic Chemistry is like climbing a ladder. You cannot skip a step to climb up or else you will fall. The reason why Coordination Compounds is an important Chapter in both Classes 11 and 12 is that both of the Chapters in these Classes are interrelated. A student is always advised to study both these Chapters but most importantly students must study the Chapter from Class 11 before solving the problems from Class 12. This will help them to score high marks.
3. Is there a way to revise the entire syllabus of Inorganic Chemistry in one go?
However, what if we told you that we have taken care of that for you? At Vedantu we believe in giving everything that our students ask for. Ask and you shall receive. So here is the link to a video that covers ALL of Inorganic Chemistry for Classes 11 and 12. Yes. ALL OF INORGANIC Chemistry.
4. How do I memorize all the reactions from the Chapter Coordination Compounds?
It is recommended to use the old-fashioned way of writing all the reactions down on a sheet of paper to memorize the most important reactions and equations from Inorganic Chemistry. Inorganic Chemistry as a whole is a little easier than Organic Chemistry and that is why scoring full marks in it is definitely possible. So make sure that you are using the method of writing down the most important formulas and reviewing them over and over after some time to make sure that the content remains fresh in your memories.
5. Can I skip a Chapter from Inorganic Chemistry?
Unfortunately, skipping a Chapter from Inorganic Chemistry is going to be very problematic for students. Using the ladder analogy again, you cannot skip a step. In order to go up, one must take every single step and since Inorganic Chemistry has so many interconnected Chapters, skipping any one of them makes the understanding of the next one difficult. So be smart and make sure to study everything from Inorganic Chemistry. Skipping any Chapter is not recommended if students want to score well!

























