




How Mendeleev’s Table Revolutionized Chemical Classification
The framework for the modern periodic table was developed in 1869 by Russian chemist Dmitri Ivanovich Mendeleev in response to the failure of Dobereiners' and Newlands' predictions regarding it. He proposed a scientific marvel that became a milestone in the history of classification.
At the time of Mendeleev's study, there were only 63 known elements. Mendeleev studied the characteristics of each element and found that the qualities were periodically related to atomic mass. He observed the arrangement of elements in ascending order of atomic mass and the periodic recurrence of elements with similar properties. He organised the elements in the periodic table so that those with similar properties fell into the same vertical columns as those in the same horizontal rows. The horizontal rows are named "periods," and the vertical columns are known as "groups."
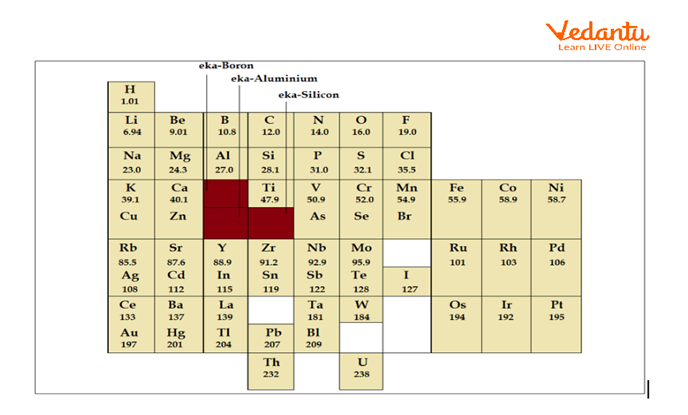
Mendeleev’s Periodic Table
Mendeleev’s Periodic Law
According to Mendeleev's periodic law, an element's atomic weight is a periodic function of the element's physical and chemical properties. Mendeleev postulated that elements' periodic physical and chemical properties rely on their atomic weight, such that when they are arranged in increasing order of atomic weight, elements with similar properties are repeated at regular intervals of increasing atomic weight.
Main Features of Mendeleev Periodic Table
At the time Mendeleev started his work, there were 63 known elements. He reacted each element with Hydrogen and Oxygen to form compounds. He chose these gases as they react with most of the elements. Upon reaction, he discovered that some elements and their compounds had similar properties.
For instance, Lithium and Sodium. He observed that both these elements were soft and shiny. Both lithium and sodium form oxide in reaction with oxygen. They both also react with water to produce hydroxides. Because of these similar properties, lithium and sodium were grouped together.
Mendeleev placed such groups of elements in a table. Inside the group, he arranged them in order of increasing atomic mass. He observed that elements in the same group appeared after a fixed number of elements. Like over sodium is placed below lithium after a gap of 6 elements. In the same way, potassium appears below sodium after a gap of 6 elements.
He also noticed that by doing so, elements were automatically getting arranged in order of increasing atomic masses. This repeating pattern of chemical properties when elements are arranged in order of their atomic masses is a periodic or recurring event.
What are the Achievements of Mendeleev's Periodic Table?
Mendeleev left some gaps in the periodic table. He anticipated that more elements would be discovered in the near future. Thus, he left gaps in the periodic table for such elements.
He also predicts the properties of those undiscovered elements. He named these elements Eka-boron, Eka-aluminium, and Eka-silicon. The prefix "Eka" is of scientific origin and means "one".
Each of these future elements would resemble the ones placed above them on the table.
Later, he discovered elements such as scandium, gallium, and germanium show properties similar to eka-boron, eka-aluminium, and eka-silicon. As a result, scandium, gallium, and germanium have taken the place of space.
Noble gases were not discovered during that time. But once it was discovered, they could be accommodated in the periodic table without disturbing the existing array of elements.
Mendeleev observed that in some places where heavier elements come before lighter elements. He wants to ensure that elements with similar chemical properties always stay together.
These are all facts formulated on the basis of the Mendeleev periodic table.
Limitations
Between any two consecutive elements, the mass difference was not fixed, and it varied a lot. For instance, cadmium and tin differ in their masses by 6 units, and indium is present between them. On the other hand, antimony and tellurium also differ by a mass of 6 units, but there are no elements present in between them.
Isotopes were discovered a long time after this proposal of the periodic table. Since their masses differed only by small amounts, it was impossible to place them in the same group, despite having a similar chemical nature.
Hydrogen forms compound like HCl, which resemble compounds like NaCl formed by alkali metals. On the other hand, hydrogen resembles halogens since both exist as diatomic molecules (H2, Cl2, etc.). They also form similar kinds of covalent bonds (CH4, CCl4, etc.). As a result, there is an anomaly in the position of hydrogen. This was not explained by Mendeleev.
Despite these limitations, Mendeleev's periodic table was the first successful classification of its time.
Interesting Facts
The role of Mendeleev's periodic table in the formation of the modern periodic table is inevitable. He developed his periodic table in the order of atomic weights. To find the atomic weight of each element, he allows an electric current to pass through a solution containing those atoms to be weighed. The current may seem to break the solution into atoms of specified elements. While using batteries, it is easy to separate atoms as they show polarity differences. The atoms will be separated differently based on their polarity.
Mendeleev has a strong passion for playing card games. He sorted the cards according to how they would arrange in a solitaire game after writing the weight of each element on a separate index card.
Key Features
In Mendeleev's periodic table, the elements are arranged vertically and horizontally on the basis of their properties. The vertical columns are called groups, and the horizontal rows are called periods. Along with that, Mendeleev's periodic table has the following advantages:
It includes blank spaces for undiscovered elements and also predicts the properties of those undiscovered elements.
It has provisions for undiscovered noble gases.
But once it was discovered, they could be accommodated in the periodic table without disturbing the existing array of elements.
Make certain that elements with similar properties stay together.
FAQs on Key Achievements of Mendeleev’s Periodic Table
1. What distinguishes Mendeleev's periodic table from the modern periodic table?
The modern periodic table is based on atomic number, in contrast to Mendeleev's periodic table, which is based on atomic mass. Mendeleev's work has just 63 elements, but the modern periodic table has 118. Mendeleev left certain places empty for the elements that had not yet been found.
On the other hand, the current periodic table is uniform and free of these gaps. Noble gases were not identified during Mendeleev's study. Noble gases are positioned in the 18th group of the modern periodic table without affecting the order of Mendeleev's periodic table.
2. Explain Dobereiner's triads of elements.
Johann Dobereiner was the first to classify elements and discover similar triads of elements, which led to the development of the periodic table of elements. He started classifying elements in the increasing order of their atomic masses. He found that a group of three elements with similar chemical properties can be obtained.
He also found that the atomic mass of the middle element is always equal to the arithmetic mean of the atomic masses of the other two elements. For instance, the arithmetic mean of the masses of potassium and lithium corresponds to 23.02, almost equal to sodium's atomic mass (22.99).
3. How did Lother Maeyer contribute to the development of the periodic table?
of atomic mass and density. He plotted his findings into a curve, and named it the Lother Meyer atomic volume curve. It is a plot of atomic mass and atomic volume on the X and Y-axis, respectively. This curve consists of sharp peaks and broad minima. He explained that elements with similar properties occupy similar positions on this curve.
For example, alkali metals occupy the peaks of the curve, which means that alkali metals have a higher atomic volume. On the basis of these observations, Lother Maeyer proposed that the physical properties of the elements are a periodic function of their atomic masses.
























