




How Common Salt Forms Essential Industrial Chemicals
Salt is a chemical that is created when a metal is used to replace the acid's hydrogen. For instance, when Na is substituted for H in HCl, NaCl results. When an acid and a base react, salts are created. It is basically a neutralisation reaction that occurs when an acid and a base react. Salts are primarily solids, have high melting and boiling points, and conduct electricity when dissolved in water. All salts having the same positive ions are considered to belong to the same family of salts.
Table salt is made up of the elements sodium (Na) and chloride (Cl). Both elements are found linked together in nature as the compound sodium chloride rather than occurring independently and freely.
Common salt is made from seawater that has evaporated and is majorly extracted by the process of crystallisation of the brine solution.
Different Chemical Products Obtained from Common Salt
Common salt can serve as the raw material for the production of sodium hydroxide. Hydrogen gas and chlorine gas are the byproducts of the process. Baking soda or sodium hydrogen carbonate, washing soda or sodium carbonate decahydrate and bleaching powder or calcium hypochlorite can also be obtained as a product of common salt. Common salt can be used as a preservative for pickles and for preparing food. Common salt is employed in the production of soap and in the melting of ice.
How is Salt Obtained from Seawater?
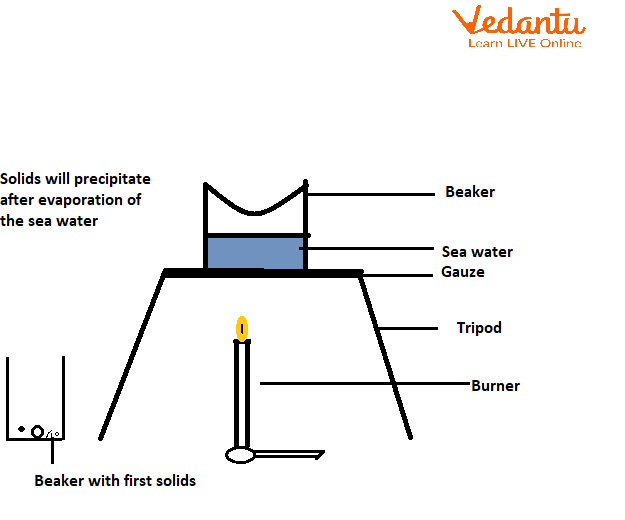
Large-scale sources of sodium chloride include common salt that is made from seawater. To obtain the salt seawater is first trapped in big shallow pools and left to stand. The common salt is left behind as the water gets slowly evaporated into vapour under the heat of the sun.
The obtained common salt is then purified from its impurity by the process of crystallisation. Crystallisation is the process of purification of a compound from a concoction. For this, the substance is heated until it dissolves in a solvent or mixture of solvents. By letting some of the solvents evaporate, the mixture is concentrated. Crystals of the pure chemical are precipitated due to cooling the solvent. The final product is obtained by filtration followed by drying. Hence, salt is obtained from seawater by the process of evaporation and it is a physical change.
When the temperature rises, a liquid transforms into its gaseous state, which is known as evaporation. High energy molecules leave the liquid's surface during evaporation, which has a cooling effect. Sea and ocean water both contain significant amounts of salt. It is created when sodium and chlorine ions mix in water. This salt is frequently used for cooking and other purposes.
Rock salt is a common salt that has been extracted from subsurface sources. Rock salt is composed of huge crystals of common salt. Similar to coal, it is removed from an underground deposit.
Process of Obtaining Salt From Seawater
Alternative Process of Obtaining Salt from Seawater
Reverse osmosis is a method, in which the water is allowed to pass through a permeable filter eventually increasing the concentration of salt as the water is pushed out. This method is very expensive and can be used to separate salt for a few Litres of water only.
Another popular method is Electrodialysis. Water is poured into a big container in this process which involves adding a porous membrane between a negatively charged anode and a positively charged cathode. The anode then draws the positive sodium ions when the electric current is transferred, whereas the cathode attracts the negative chloride ions leaving water behind. As a result, salt and water are separated. Salt can also be obtained from sea water chemically. The seawater and decanoic acid are combined in a container, and the mixture is then heated. The solution is cooled after a while. The salt is formed during cooling and settles to the bottom of the container as precipitates. Decanoic acid and water combine to generate two layers that can be removed individually.
Interesting Facts
Salt, often known as sodium chloride (NaCl), has 60% chloride and 40% sodium by weight. Sodium, an essential mineral found in salt, serves as the body's main electrolyte.
Common salt is very important in our daily life because all signals to and from the brain, as well as those that travel within the cells, depend on sodium to function.
Key Features of Chemicals from Common Salt
Common salt has a lot of intriguing characteristics. It is white, transparent crystalline powder and colourless.
It is obtained from seawater by the process of evaporation which is a physical change.
The main sources of ocean salt are seafloor openings and rocks on land. The main source of salts dissolved in seawater is the rock on land. Due to its moderate acidity, rainwater that falls on land erodes rocks.
FAQs on Chemicals From Common Salt Explained: Types, Uses & Benefits
1. What are the major chemicals produced from common salt (Sodium Chloride)?
Common salt (Sodium Chloride, NaCl) is a crucial raw material for producing several important chemicals as per the CBSE Class 10 syllabus. The primary chemicals derived from it include:
- Sodium Hydroxide (NaOH), also known as caustic soda.
- Sodium Carbonate (Na₂CO₃·10H₂O), commonly called washing soda.
- Sodium Bicarbonate (NaHCO₃), also known as baking soda.
- Bleaching Powder (CaOCl₂).
- Chlorine (Cl₂) and Hydrogen (H₂) gases, which are co-products of sodium hydroxide production.
2. What is the chlor-alkali process and why is it named so?
The chlor-alkali process is the industrial method used to produce sodium hydroxide (NaOH) by the electrolysis of an aqueous solution of sodium chloride (brine). It is named 'chlor-alkali' because the products formed are 'chlor' for chlorine gas (Cl₂) and 'alkali' for the sodium hydroxide (NaOH), which is a strong alkali. Hydrogen gas (H₂) is also produced as a by-product.
3. What is washing soda, and what are its main uses?
Washing soda is the common name for hydrated sodium carbonate, with the chemical formula Na₂CO₃·10H₂O. It is a crystalline solid that is soluble in water. Its main uses include:
- As a cleaning agent in laundry.
- For removing the permanent hardness of water.
- In the manufacturing of glass, soap, and paper.
- As a laboratory reagent in various chemical processes.
4. How is bleaching powder (Calcium Oxychloride) produced and what is its primary function?
Bleaching powder (CaOCl₂) is produced by the action of chlorine gas (obtained from the chlor-alkali process) on dry slaked lime [Ca(OH)₂]. The chemical reaction is: Ca(OH)₂ + Cl₂ → CaOCl₂ + H₂O. Its primary function is as a bleaching agent for whitening cotton and linen in the textile industry and wood pulp in paper factories. It is also a powerful disinfectant used for treating drinking water.
5. What is the chemical difference between baking soda and baking powder?
The key chemical difference lies in their composition. Baking soda is a single compound, sodium bicarbonate (NaHCO₃). In contrast, baking powder is a mixture containing baking soda (sodium bicarbonate), a mild edible acid like tartaric acid, and a neutral substance like starch to prevent a premature reaction. Baking soda requires an external acidic ingredient to react, while baking powder contains both the base and the acid needed for leavening.
6. How does washing soda (sodium carbonate) soften hard water?
Hard water contains dissolved calcium (Ca²⁺) and magnesium (Mg²⁺) ions, which interfere with the cleaning action of soap. Washing soda (sodium carbonate, Na₂CO₃) softens water through a precipitation reaction. When added to hard water, the carbonate ions (CO₃²⁻) from washing soda react with the calcium and magnesium ions to form insoluble precipitates of calcium carbonate (CaCO₃) and magnesium carbonate (MgCO₃). These solid precipitates are removed from the water, thus eliminating its hardness.
7. Why is an aqueous solution of sodium chloride, known as brine, used as the starting material for so many important chemicals?
Brine, an aqueous solution of sodium chloride (NaCl), is an ideal starting material because it is abundant, inexpensive, and contains the essential sodium (Na⁺) and chloride (Cl⁻) ions. Through the process of electrolysis (the chlor-alkali process), these stable ions are converted into highly reactive and useful products like chlorine gas (Cl₂), hydrogen gas (H₂), and a solution of sodium hydroxide (NaOH). These products then serve as versatile building blocks for manufacturing a wide range of other chemicals.
8. The chlor-alkali process produces three useful products. Why is it important to keep them separate during production?
It is crucial to keep the three products of the chlor-alkali process—chlorine (Cl₂), hydrogen (H₂), and sodium hydroxide (NaOH)—separate for two main reasons. Firstly, for utility, as each product has distinct industrial applications that would be compromised if mixed. Secondly, for safety, as chlorine and hydrogen gases can react explosively to form hydrogen chloride (HCl). Furthermore, if chlorine gas mixes with the sodium hydroxide solution, it reacts to form sodium hypochlorite (bleach), preventing the collection of pure NaOH and Cl₂.
9. Besides its use in baking, what makes baking soda (sodium bicarbonate) an effective antacid?
Baking soda (sodium bicarbonate, NaHCO₃) is an effective antacid because it is a mild, non-corrosive base. The stomach contains hydrochloric acid (HCl) to aid digestion. When there is excess acid, it can cause indigestion and heartburn. As a basic salt, baking soda reacts with and neutralises the excess stomach acid, converting it into harmless salt (NaCl), water (H₂O), and carbon dioxide (CO₂), providing quick relief. The reaction is: NaHCO₃ + HCl → NaCl + H₂O + CO₂.






































