




What is DMP Reagent? Structure, Mechanism & Key Applications
DMP's full form is Dess Martin Periodinane, which is commercially accessible and decomposes slowly. However, DMP is heat- and shock-sensitive and shows an exotherm once heated >130 °C. DMP chemical may be a widely used chemical agent for the mild oxidation of alcohols to aldehydes and ketones. The neutral condition of the oxidation reaction makes DMP an appropriate selection in syntheses of sensitive, functionally complex intermediates. DMP shows several merits over other common oxidative agents like Cr and DMSO-based oxidants; so, it's habitually used in the total synthesis of natural products.
DMP Reagent
Dess-Martin periodinane (DMP), a commercially accessible chemical, is often used as a mild oxidative agent for the selective oxidation of primary and secondary alcohols to their corresponding aldehydes and ketones, respectively.
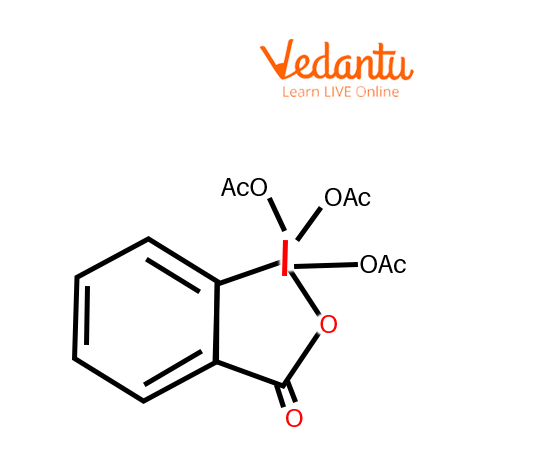
Dess Martin Reagent
DMP could be a relatively mild chemical agent that may be used under neutral conditions providing a high degree of selectivity and functional group compatibility. Other advantages include the fact that DMP is far less harmful than Cr-based alternatives and therefore, the work-up is comparatively easy. One of the main drawbacks is the potential explosive capability of DMP. The cost of DMP is also comparatively high and its high molecular weight typically necessitates using quite a lot of chemical agents.
Dess Martin Periodinane Oxidation
Alcohols can be oxidised to aldehydes, ketones, and carboxylic acids depending on their structure and also the type of oxidant used. Oxidising agents are typically classified as strong and mild oxidising agents. Mild oxidising reagents stop the oxidation of the alcohol once the carbonyl group is formed. And if it's a primary alcohol, the product formed is an aldehyde whereas the oxidation of secondary alcohol leads to ketone formation. The strong oxidising agents oxidise primary alcohols to carboxylic acids and secondary alcohols to ketones.
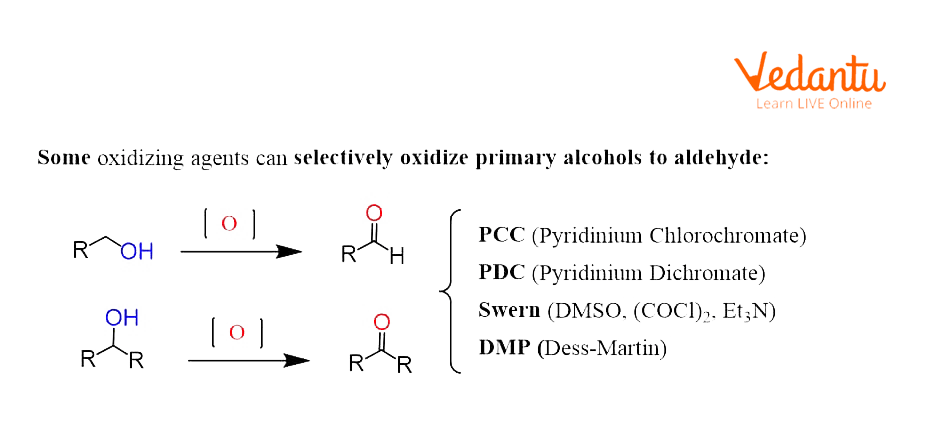
Oxidation of Alcohol Using DMP As an Oxidising Agent
Mechanism of Dess Martin Oxidation
Dess–Martin periodinane (DMP oxidation) may be a selective methodology for oxidising primary alcohols to aldehydes.
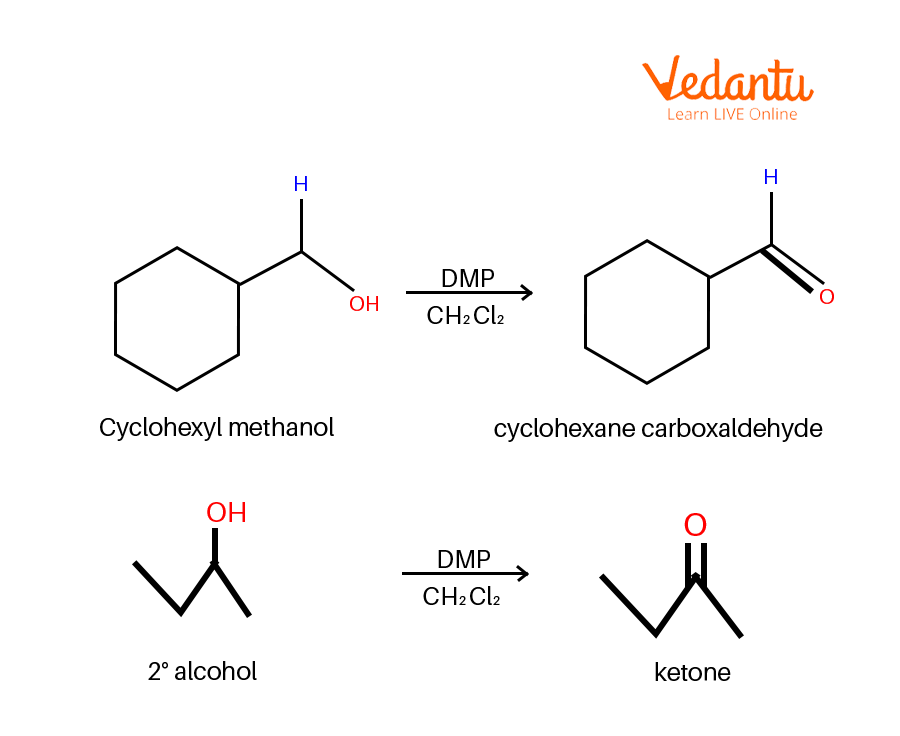
Dess Martin Oxidation Example
Another advantage of using the DMP chemical as an oxidant is that it can be performed under milder conditions and does not need the presence of strong acids. The reaction starts with the substitution of the iodine where the alcohol replaces one of the acetate ions followed by deprotonation that forms an aryl iodo(III) ester–periodinane intermediate.
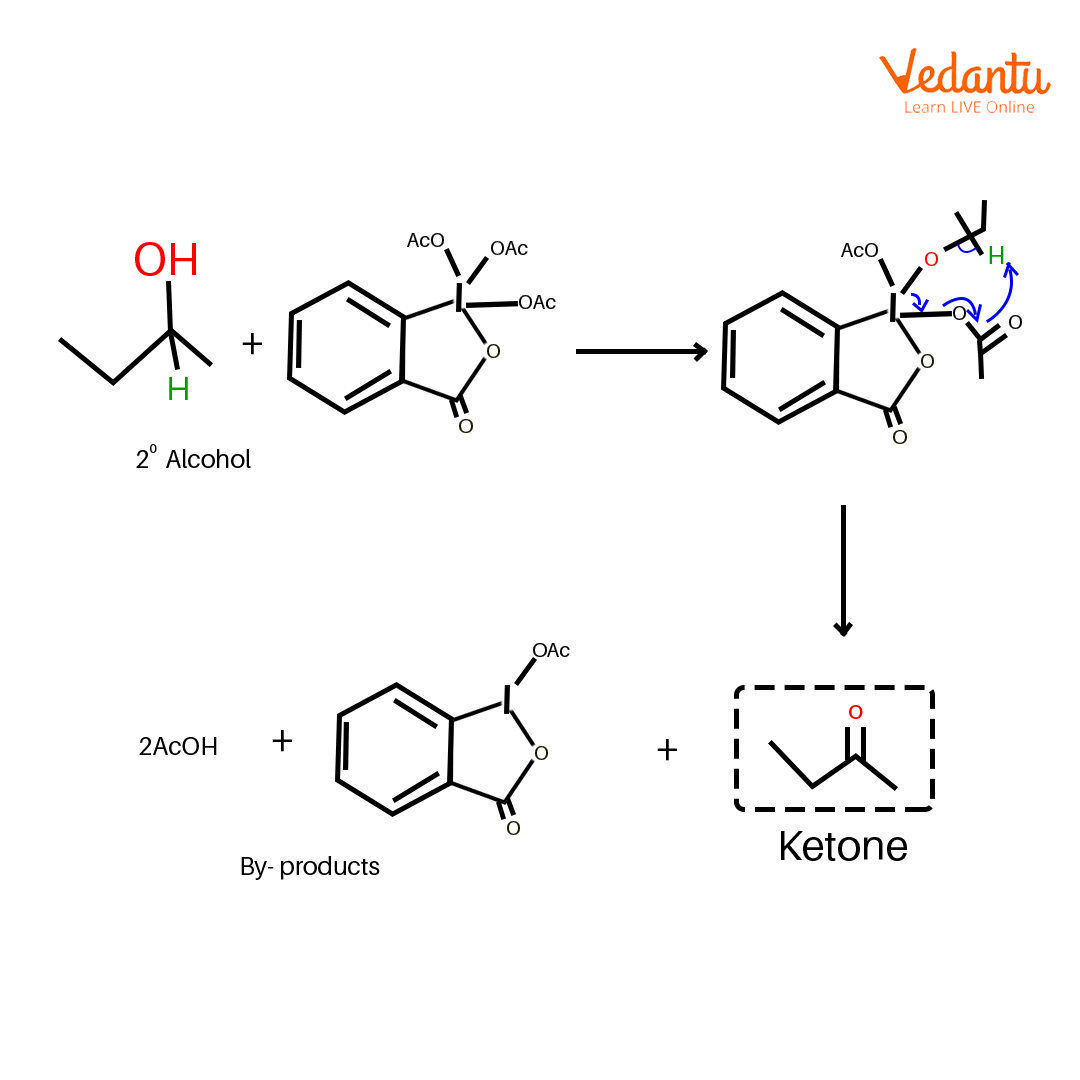
Mechanism of Dess Martin Oxidation
The periodinane intermediate is then transformed to the corresponding carbonyl compound by attainable intramolecular removal of the ɑ-hydrogen to form a C=O π bond.
Preparation of DMP Reagent
Dess-Martin Periodinane (DMP) is an acylated variant of the oxidant IBX. The acyl group provides DMP additional solubility in organic solvents than IBX. The most friendly synthesis of IBX has been determined to be treating 2-iodobenzoic acid with oxone in water, at elevated temperatures for three hours.

Preparation of DMP Reagent
Interesting Fact
It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the chemical agent in 1983.
It is based on IBX; however, because of the acetate groups connected to the central iodine atom, DMP is far more reactive than IBX and is much more soluble in organic solvents.
Important Question
1. What does DMP do to primary alcohol?
Ans: DMP is a chemical agent used to oxidise primary alcohols to aldehydes and secondary alcohols to ketones.
2. What is IBX in organic chemistry?
Ans: 2-Iodoxybenzoic acid (IBX) is a chemical compound used in organic synthesis as an oxidising agent. This periodinane is particularly suited to oxidise alcohols to aldehydes.
Conclusion
Dess–Martin periodinane (DMP) may be a hypervalent iodine reagent used to oxidise primary alcohols to aldehydes and secondary alcohols to ketones. This chemical agent is enticing because of its operation under mild conditions (room temperature, neutral pH), short reaction times, higher yields, high chemoselectivity, tolerance of sensitive functional groups, and long life on the shelf. The presence of acetate groups makes DMP more soluble in a very wider range of solvents compared to the IBX chemical agent.
FAQs on DMP Reagent: Definition, Mechanism & Uses
1. What is DMP Reagent and what is its primary use in organic chemistry?
DMP Reagent, or Dess-Martin Periodinane (DMP), is a hypervalent iodine compound used as a mild and selective oxidising agent. Its primary function in organic chemistry, as per the CBSE syllabus, is to oxidise primary alcohols to aldehydes and secondary alcohols to ketones under neutral conditions, making it ideal for synthesising complex and sensitive molecules.
2. How is DMP Reagent prepared?
DMP Reagent is prepared from its precursor, 2-Iodoxybenzoic acid (IBX). The synthesis involves treating 2-iodobenzoic acid with an oxidising agent like oxone in water to produce IBX. The IBX is then acylated, typically with acetic anhydride, which replaces the hydroxyl groups with acetate groups to form DMP. This modification is important because it significantly improves the reagent's solubility in organic solvents.
3. What is the mechanism of alcohol oxidation using DMP Reagent?
The mechanism for oxidation by DMP Reagent involves two main steps. First, the alcohol attacks the iodine atom, replacing one of the acetate ligands in a substitution reaction. This is followed by deprotonation to form a periodinane intermediate. In the second step, an intramolecular elimination occurs where the α-hydrogen from the alcohol moiety is removed, leading to the formation of the C=O double bond of the final carbonyl compound (an aldehyde or ketone).
4. Why is DMP Reagent often preferred over other oxidising agents like chromium-based reagents?
DMP Reagent is often preferred over other oxidants like those based on chromium (e.g., PCC, PDC) for several key reasons important for complex syntheses:
- Mild Conditions: The reaction proceeds at room temperature and neutral pH, protecting sensitive functional groups elsewhere in the molecule.
- High Selectivity: It selectively oxidises alcohols without the common problem of over-oxidising primary alcohols to carboxylic acids.
- Reduced Toxicity: It is significantly less toxic and environmentally harmful than chromium-based reagents.
- Simple Work-up: The process to isolate the product after the reaction is generally more straightforward.
5. What is the main difference between DMP Reagent and its precursor, IBX?
The main difference between DMP Reagent and its precursor, IBX (2-Iodoxybenzoic acid), lies in its structure and resulting properties. DMP has acetate groups attached to the central iodine atom, whereas IBX has hydroxyl groups. This structural change makes DMP much more soluble in common organic solvents like dichloromethane. This enhanced solubility also contributes to DMP being generally more reactive and easier to use in a wider range of reaction conditions than IBX.
6. Which solvents are typically used for oxidation reactions with DMP Reagent?
Oxidation reactions using DMP Reagent are typically carried out in halogenated organic solvents. The most common choices mentioned in synthetic procedures are dichloromethane (CH₂Cl₂) or chloroform (CHCl₃). These solvents are effective because DMP is highly soluble in them, allowing the reaction to proceed efficiently at or near room temperature.
7. What are the potential drawbacks or limitations of using DMP Reagent?
Despite its advantages in the lab, using DMP Reagent has some significant drawbacks that limit its application, particularly on a larger scale:
- Explosive Potential: DMP is sensitive to heat and shock. It can decompose exothermically when heated above 130 °C, posing a safety risk.
- High Cost: The reagent is relatively expensive, which makes it less economically viable for industrial-scale chemical production.
- Poor Atom Economy: Due to its high molecular weight, a large mass of the reagent is required relative to the substrate, generating significant waste.
























