




What Are Internal Alkynes? Definitions, Examples, and Identification
The simplest alkyne, a hydrocarbon with a triple bond connecting carbon atoms, has the chemical formula C2H2 and is more often referred to as acetylene. Its skeletal system is H-C≡C-H.
Alkynes are a chemical compound. Alkynes are molecules that have a triple bond between two carbon atoms. The triple bond is composed of one bond and two bonds.
Alkynes are nonpolar, unsaturated hydrocarbons that have comparable physical characteristics as alkanes and alkenes. Alkynes are soluble in organic solvents, somewhat soluble in polar solvents, and insoluble in water. Alkynes have somewhat higher boiling points than alkanes and alkenes.
Physical Properties of Alkyne
Unsaturated hydrocarbons with at least one triple bond between carbon atoms are known as alkynes. Alkynes are classified into two types: terminal and internal. Terminal alkynes are triple-bonded compounds that share a triple bond with the carbon at the end of the chain.
Internal alkynes are compounds with a triple bond between two carbon atoms that are not terminal. Alkynes have the typical molecular formula CnH2n-2. Alkynes' physical characteristics are extremely similar to alkenes' physical qualities.
The hybridisation is responsible for the alkyne structure's distinctiveness. The triple bonds in alkynes are responsible for their acidity, non-polar bonding strength, and linearity.
These chemicals are soluble in polar solvents but completely insoluble in water. Alkynes can dissolve in organic solvents because the density of the solution is lower, which is also a property of alkenes. It is, for example, capable of dissolving in an ether solution.
Structure and Bonding of Alkyne
The H-C≡C bond angles in acetylene are 180°. Alkynes are rod-like due to their bond angle. Cyclic alkynes are also uncommon. It is impossible to separate benzyne. The C≡C bond distance of 121 picometers is substantially less than the C=C distance or the C-C bond distance in alkenes or alkanes.
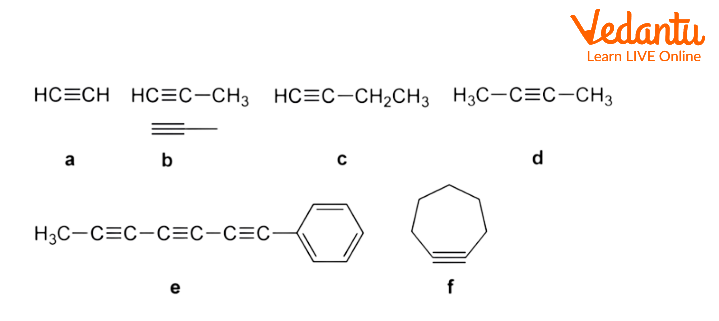
In the above figure, all data are as follows:
acetylene, b, two representations of propyne, c, 1-butyne, d, 2-butyne, e, naturally occurring 1-phenylhepta-1,3,5-triyne, and f, strained cycloheptyne. Blue highlights triple bonds.
The sigma bond contributes 369 kJ/mol of bond strength, the first pi bond contributes 268 kJ/mol of bond strength, and the second pi-bond gives 202 kJ/mol of bond strength. Bonding is often described in the context of molecular orbital theory, which identifies the triple bond as the result of s and p orbital overlap. The carbon atoms in an alkyne bond are sp hybridised in valence bond theory: they each contain two unhybridized p orbitals and two sp hybrid orbitals.
Each atom's sp orbital overlaps to produce one sp-sp sigma bond. Each p orbital on one atom overlaps one on the other, resulting in the formation of two pi bonds, for a total of three bonds. Each atom's leftover sp orbital can create a sigma bond with another atom, such as hydrogen atoms in the parent acetylene. The two sp orbitals are positioned on opposing sides of the carbon atom.
Nomenclature of Alkyne
Alkynes are designated using the Greek prefix method, with no extra letters, in systematic chemical nomenclature. Ethyne and octyne are two examples. In parent chains containing four or more carbons, the position of the triple bond must be specified.
When the bond begins at the third carbon, octyne might be written as 3-octyne or oct-3-yne. When there are no better functional groups, the parent chain must include the triple bond, even if it is not the longest carbon chain feasible in the molecule. Ethyne is sometimes known colloquially as acetylene.
In Chemistry, the suffix -yne denotes the presence of a triple bond. In organic chemistry, suffixes frequently follow IUPAC nomenclature. However, inorganic compounds containing unsaturation in the form of triple bonds can be designated via substitutive nomenclature in the same way as alkynes are (that is, the equivalent saturated compound's name is changed by replacing the "-ane" ending with "-yne"). When there are two triple bonds, "-diyne" is used, and so on.
A number locant immediately preceding the "-yne" suffix, or 'locants' in the case of numerous triple bonds, indicates the position of unsaturation. The number of locants is kept as low as feasible. The suffix "-yne" is also used as an infix to refer to substituent groups that are trivalently bonded to the parent molecule.
Reactions of Alkynes
Alkynes engage in numerous organic processes because they have a reactive functional group. Because alkynes are more unsaturated than alkenes, they exhibit reactions that indicate they are "doubly unsaturated." Alkynes can contribute two equivalents of H2, whereas alkenes can only add one equivalent. Alkynes contribute one or two equivalents of hydrogen depending on the catalysts and circumstances. Because alkanes are less valuable, partial hydrogenation, which stops after adding only one equivalent to give the alkene, is frequently preferred:
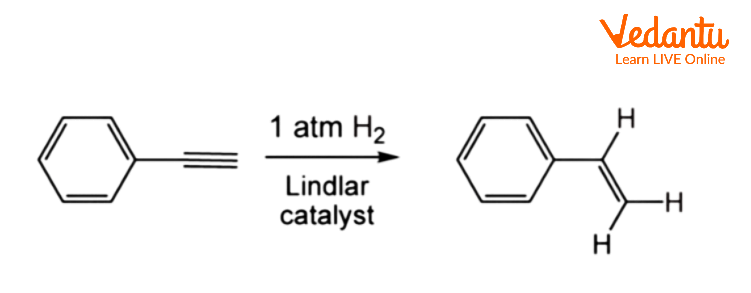
Similarly, alkynes can be halogenated to produce alkene dihalides or alkyl tetrahalides:
$RC \equiv CR' + {H_2}\xrightarrow{{}}cis - RCH \equiv CR'H$
$RCH = CR'H + {H_2}\xrightarrow{{}}RC{H_2}CR'{H_2}$
Cis-alkenes are formed by adding one equivalent of H2 to internal alkynes.
Alkynes can add two equivalents of halogens and hydrogen halides,
$RC \equiv CR' + 2B{r_2}\xrightarrow{{}}RCB{r_2}CR'B{r_2}$
Acetophenone is produced through the hydration of phenylacetylene, and the (Ph3P)AuCH3-catalysed hydration of 1,8-nonadiyne to 2,8-nonanedione:
$PhC \equiv CH + {H_2}O\xrightarrow{{}}PhCOC{H_3}$
$HC \equiv C{(C{H_2})_5}C \equiv CH + 2{H_2}O\xrightarrow{{}}C{H_3}CO{(C{H_2})_5}COC{H_3}$
Synthesis
Dehydrohalogenation is used to create alkynes from dihaloalkanes. The bulk of these reactions occurs at high temperatures and with alkoxide bases (other strong bases can also be employed).
As a result of this combination, the E2 mechanism produces the bulk of the product. Remember that the E2 process is a coordinated response (occurs in 1 step). However, the molecule undergoes three distinct transformations in one single process. This is the result of the reaction between 2-bromo-2-methylpropane with sodium hydroxide.

When we apply this notion to two halides on vicinal or geminal carbons, the E2 reaction occurs twice, resulting in the production of two pi bonds and an alkyne, as illustrated in the instances below, where the strong base is denoted by the letter B-.
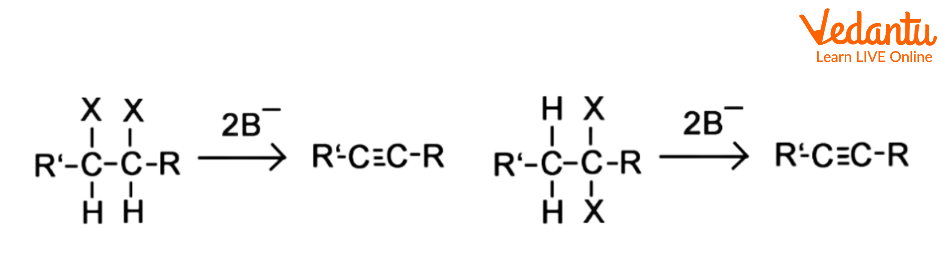
A Vicinal Dihalide's E2 is doubled.
Because of the greater acidity of alkynes, which is explained in a later part of this chapter, the reaction of terminal haloalkanes takes three equivalents of base rather than two.
The mechanism of a reaction between 2,3-dibromopentane and sodium amide in liquid ammonia is depicted here, where liquid ammonia does not participate in the reaction but is employed as a solvent.
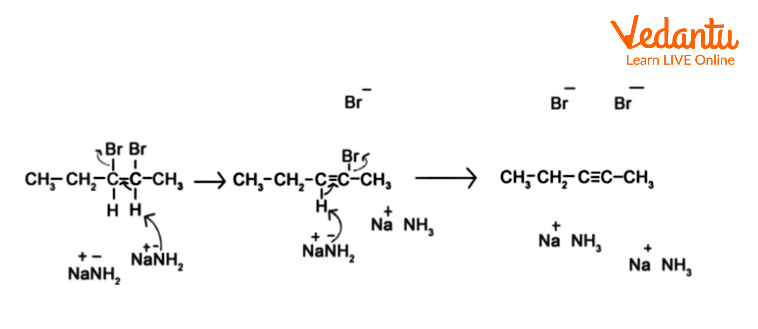
Take note of the intermediate in the alkyne synthesis. It is stereotypically in its opposite form. Because the second proton and halogen are removed from the molecule, this has no bearing on alkyne production.
Important Questions
1. Trans alkenes are formed when alkynes are reduced with sodium in liquid ammonia. Will the butene generated as a result of the reduction of the 2-butyne exhibit geometrical isomerism?
Answer: Yes, butene generated by reducing 2-butyne exhibits geometrical isomerism.
But-2-ene is generated when but-2-yne is reduced, with both methyl groups on the same or opposite side to display geometrical isomers.
2. How are you going to separate propene from propyne?
Answer: When propyne reacts, the mixture is passed through an ammoniacal AgNO3 solution while propene passes over.
3. Which of ethylene and acetylene is more acidic, and why?
Answer: Acetylene. Ethylene and acetylene both feature sp2, sp hybridised C atoms. Acetylene is more acidic than ethene because its C-H bond has a 50% S character rather than ethene's 33% S-Character.
Conclusion
Each acetylenic carbon in the internal alkynes has a carbon substituent. 3-hexyne and diphenylacetylene are two symmetrical instances. The formula for terminal alkynes is RC2H.
An alkyne is an unsaturated carbon with at least one carbon-carbon triple bond in organic chemistry. The simplest acyclic alkynes are those with only one triple bond and no additional functional groups attached, and they form a homologous series with the general chemical formula CnH2n-2.
Alkynes are commonly referred to as acetylenes, while the term acetylene also refers to C2H2, also known as ethyne in IUPAC nomenclature. Alkynes, like other hydrocarbons, are hydrophobic.
FAQs on Internal Alkynes: Structure, Properties, and Uses
1. What distinguishes alkynes?
Because alkynes contain only carbon and hydrogen, they are nonpolar and, like alkanes and alkenes, are not soluble in water. They are also less dense than water.
Alkanes are saturated hydrocarbons because they only have one link between the carbon atoms. Alkenes have at least one double bond between carbon atoms. Alkynes have one or more triple bonds between carbon atoms. Unsaturated hydrocarbons are the name given to alkenes and alkynes. While alkenes do not, alkynes produce a white precipitate with an ammoniacal solution of silver nitrate.
2. What is the primary use of alkyne?
Some of these alkynes are converted into chemical compounds such as ethanoic acid, acrylic acid, and ethanol. Ethyne is most typically utilised in the production of organic molecules such as ethanol, ethanoic acid, and acrylic acid. It is also employed in the production of polymers and polymer raw materials. Alkenes have a variety of uses in manufacturing. They serve as the raw ingredients for the synthesis of alcohols, polymers, lacquers, detergents, and fuels. Ethene, propene, and 1,3-butadiene are the three most significant alkenes for the chemical industry.
3. Which alkene has the highest stability?
Because 3-methylpent-2-ene has the most alkyl groups attached to all the supplied alkenes, it is the most stable alkene. Because it is more substituted, an internal alkyne is more stable than a terminal alkyne. An internal alkyne's heat of hydrogenation is lower than a terminal alkyne's heat of hydrogenation. Every hydrogenation process results in the formation and dissolution of the same bonds; hence the heat of hydrogenation serves as a gauge for the stability of various alkene varieties. This implies that the alkene is more stable, the lower the hydrogenation heat.























