




How Neighbouring Group Participation Simplifies Reaction Mechanisms
In this substitution reaction, one group of the substrate participates in the reaction first and hence influences it. The response rate is raised several times as a result of NGP.
The reaction of a sulphur or nitrogen mustard with a nucleophile is a classic example of NGP; the reaction rate is substantially faster for the sulphur mustard and a nucleophile than for a primary or secondary alkyl chloride without a heteroatom. The reaction rate of Ph-S-CH2-CH2-Cl with water is 650 times quicker than CH3-CH2-CH2-Cl.
NGP by Aromatic Ring
According to molecular orbital theory, the reactivity of a benzyl halide is greater because the SN2 transition state has a comparable overlap effect to that of an allyl system.
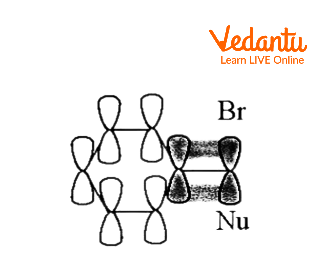
By delocalizing the positive charge, an aromatic ring can create a carbocationic intermediate known as a phenonium ion.

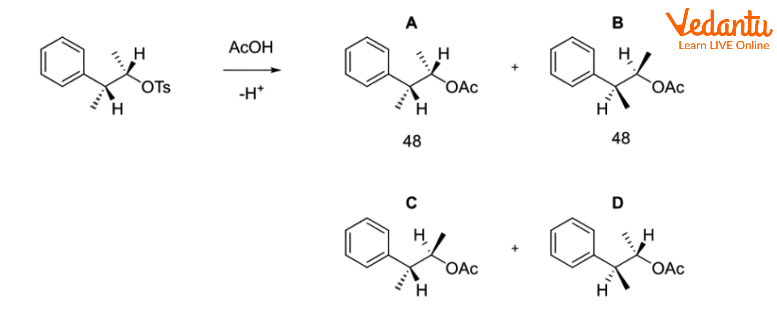
Instead of a straightforward SN2 reaction generating B when the following tosylate combines with acetic acid in solvolysis,
Working as Cyclopropane, Cyclobutane, or a Homoallyl Group
When cyclopropyl methyl chloride is combined with ethanol and water, it produces a combination of 48% cyclopropylmethyl alcohol, 47% cyclobutanol, and 5% homoallylic alcohol (but-3-enol).
This is due to the carbocationic intermediate being delocalized onto several carbons via a reversible ring opening.
NGP by an Alkane
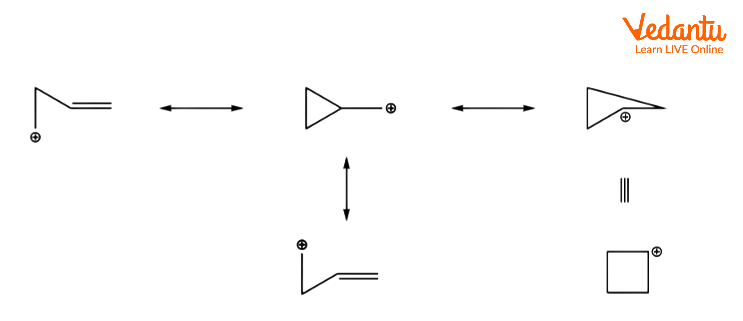
An alkene's orbitals can aid to delocalize the positive charge of a carbocation, which can help to stabilise a transition state. For example, the unsaturated tosylate will react with a nucleophile more quickly than the saturated tosylate.
The carbocationic intermediate will be stabilised by resonance, which spreads the positive charge over several atoms. This is seen in the diagram below.
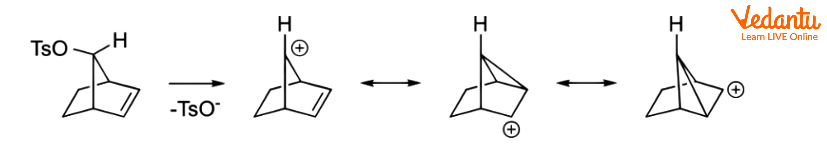
Even if the alkene is further away from the responding centre, it can still operate in this manner. In the following alkyl benzenesulfonate, for example, the alkene can delocalize the carbocation.
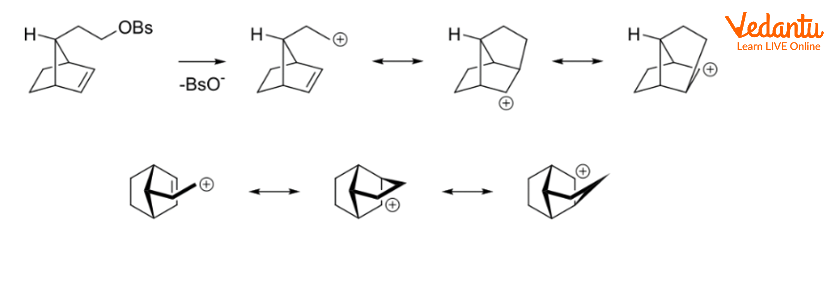
NGP by Aliphatic C–H and C–C Bonds
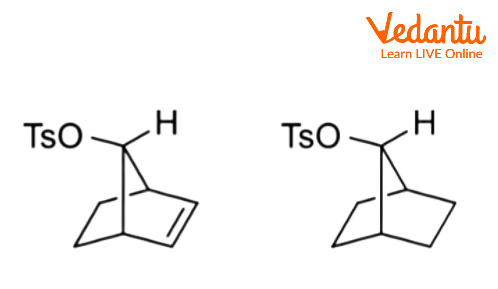
Charge delocalization can occur when aliphatic C–C or C–H bonds are near and antiperiplanar to the leaving group. Corresponding intermediates are referred to as nonclassical ions, with the 2-norbornyl system being the most well-known example.
Important Questions
1. Which following undergoes nucleophilic substitution only through the SN2 mechanism?
A. Ethyl Chloride
B. Isopropyl Chloride
D. Benzyl Chloride
Answer: (A) Ethyl chloride is the right answer.
Alkyl halides with a less bulky group linked to the halide ion favour the SN2 process. Because ethyl chloride is the least bulky of the possibilities, it will undergo nucleophilic substitution through the SN2 mechanism.
2. Which chemical is easily nucleophilic substituted?
Answer: When the leaving group stabilises and so functions as a weak base, nucleophilic substitution at acyl carbon is simple.
3. Why does benzene easily undertake electrophilic substitution reactions but struggle with nucleophilic substitutions?
Answer: Because of the presence of 6π electrons, benzene acts as a rich source of electrons, making it easily attacked by reagents lacking in electrons. As a result, benzene easily undergoes electrophilic substitution reactions but struggles with nucleophilic replacements.
4. What precisely is a regioselective reaction?
Answer: The benefit of having or breaking a chemical connection in one way over all other potential orientations is known as regioselectivity. Regioselectivity can also be used for other processes, such as adding pi-ligands. Selectivity occurs in carbene insertions, for example, in the Baeyer–Villiger reaction.
Conclusion
It is also conceivable for the reaction's stereochemistry to be aberrant (or unexpected) compared to a typical reaction. While neighbouring groups can influence many organic chemistry reactions (for example, the reaction of a diene such as 1,3-cyclohexadiene with maleic anhydride normally gives the endo isomer due to a secondary effect overlapping of the carbonyl group orbitals with the transition state in the Diels-Alder reaction), this page is limited to neighbouring group effects seen with carbocations and SN2 reactions.
(IUPAC) defines neighbouring group participation (NGP) in organic chemistry as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a pi bond contained within the parent molecule but not conjugated with the reaction centre. It is common for the reaction rate to rise when the NGP is in use.
FAQs on Neighbouring Group Participation: Key Concepts, Mechanism & Examples
1. What is Neighbouring Group Participation (NGP) in organic chemistry?
Neighbouring Group Participation (NGP), also known as anchimeric assistance, is a phenomenon where a substituent in a reacting molecule acts as an internal nucleophile. This group is positioned close to the reaction centre and assists in the departure of the leaving group, significantly influencing the reaction's rate and stereochemical outcome. It is essentially an intramolecular substitution followed by an intermolecular one.
2. What is the general mechanism of a reaction involving Neighbouring Group Participation?
The mechanism of NGP typically occurs in two distinct steps:
- Step 1 (Intramolecular Attack): A neighbouring group with a lone pair of electrons (like O, S, N, or even a π-bond) attacks the carbon atom bearing the leaving group. This is an intramolecular Sɴ2-like attack, which displaces the leaving group and forms a cyclic, bridged-ion intermediate.
- Step 2 (External Nucleophilic Attack): An external nucleophile then attacks the bridged intermediate. This attack opens the ring and leads to the final product. This step is also an Sɴ2-type reaction.
3. Why do reactions involving NGP show a significant increase in reaction rate?
Neighbouring Group Participation leads to a dramatic rate enhancement because the initial step is an intramolecular reaction. Intramolecular processes are entropically favoured over their intermolecular counterparts. The reacting groups (the internal nucleophile and the reaction centre) are already part of the same molecule, increasing the effective concentration and the probability of a successful collision, thus lowering the activation energy for the departure of the leaving group.
4. How does Neighbouring Group Participation affect the stereochemistry of the final product?
A key consequence of NGP is the overall retention of configuration at the chiral centre. This is a result of two consecutive Sɴ2 reactions, each causing an inversion of configuration. The first inversion occurs when the neighbouring group forms the bridged intermediate. The second inversion happens when the external nucleophile attacks this intermediate. The net result of these two inversions is the retention of the original stereochemistry, which is a stark contrast to a standard Sɴ2 reaction that results in a single inversion of configuration.
5. What are some common examples of atoms or groups that can act as neighbouring groups?
Several functional groups with lone pairs of electrons or π-electrons can exhibit NGP, provided they are in a suitable position (usually anti-periplanar to the leaving group). Common examples include:
- Atoms with lone pairs: Halogens (I, Br, Cl), Oxygen (in ether or hydroxyl groups), Sulphur (in thioether groups), and Nitrogen (in amine groups).
- Groups with π-electrons: A phenyl group (forming a phenonium ion intermediate) or a carbon-carbon double bond.
6. What are the essential structural requirements for Neighbouring Group Participation to occur?
For NGP to be effective, three main conditions must be met:
- Presence of a Participating Group: The molecule must contain a group with non-bonding electrons or π-electrons that can act as an internal nucleophile.
- Correct Position: The neighbouring group must be positioned correctly, typically at the γ or δ position relative to the leaving group, to allow the formation of a stable 3, 5, or 6-membered cyclic intermediate.
- Stereochemistry: The participating group must be able to attack the reaction centre from the backside (anti-periplanar), facilitating the Sɴ2-like displacement of the leaving group.
7. How is a reaction with NGP different from a standard Sɴ2 reaction?
While both involve nucleophilic attack, NGP and Sɴ2 reactions differ significantly. In a standard Sɴ2 reaction, an external nucleophile attacks the substrate in a single, concerted step, leading to an inversion of stereochemistry. In contrast, a reaction with NGP is a two-step process involving an internal nucleophile first. This results in an enhanced reaction rate and an overall retention of stereochemistry at the reaction centre.
8. Can a double bond act as a neighbouring group in a reaction?
Yes, a carbon-carbon double bond can participate as a neighbouring group if it is suitably located to attack the reaction centre. The π-electrons of the double bond can act as the internal nucleophile, displacing the leaving group to form a non-classical carbocation intermediate. This type of participation is often observed in the solvolysis of substrates like anti-7-norbornenyl tosylate, where the reaction rate is significantly faster than its saturated counterpart due to this assistance.
























