




Why Orbital Overlap Matters in Chemical Bonding
Ionic interactions and covalent bonding are viewed as 2 basic concepts for the chemical bond in today's knowledge of the phenomenon. Ionic bonds include the traditional electrostatic interactions between point charges. Electron-sharing or donor-acceptor bonds, which involve two orbitals that are profoundly populated and one that is vacant, are the two most common ways covalent bonding is described.
Even though Pauli's repulsion is greater than the electrostatic repulsive force when the orbital overlap concept increases, both types of bond formations ignore interactions among electrons with the same spin. The involved atom sizes, valence electrons, and degree of orbital overlap are the distinguishing factors. Furthermore, a larger overlap leads to a relatively strong bond formation among the two atoms. Thus, the orbital overlap theory explained the combination of atoms by interfering with one another's orbitals, resulting in relatively low energy levels where valence electrons come together to constitute covalent bonds.
What is the Concept of Orbital Overlap?
An orbital overlap is the combination of orbitals by the collision of neighbouring atoms in the space of the same area, which occurs in chemical bonds leading to a bond formation facilitated by orbital overlap. The two atoms that are close to one another, penetrate each other's orbitals during the orbital process, creating a new hybridised orbital through which the electrons of the bonding pair are located. Because it is less energetic than the atomic orbital, this hybridised orbital is firm and has a low energy state. Thus, the partial fusion of the orbital explains the orbital overlap concept.
To further clarify the system, it should be noted that it takes place over an atomic orbital. An atomic orbital is a location within the atom's interior where there is a high likelihood of finding electrons. The two provided nuclei within the atoms are also drawn to one another by the enhanced electron density in a small area, which reduces their repulsive forces.
For example, a covalent bond between H and Cl is the end outcome of the response.
Orbital Overlap Theory
Chemical bonding and the atom's shape or geometry are governed by how orbitals are arranged, which are explained by the below two orbital overlap theories. Molecular orbital theory (MOT) or Valence Bond Theory (VBT) can both be utilised to describe how these orbitals are arranged. The VBT explains the electron pair's orbital overlap. s, p, and d orbitals make up the majority of atomic orbitals.
The VBT states that a σ bond will be developed when two s or p orbitals overlap head-to-head. A π bond is created when two concurrent p-orbitals overlap. Since a double bond contains both a σ and a π bond; a single bond will comprise a σ bond. The MOT explains how overlapping atomic orbitals create molecular orbitals. This theory states that a molecular orbital can only support a maximum of 2 electrons. To reduce the attraction among them, these charged particles possess opposite spin.
Difference Between VBT and MOT
Overlap of Atomic Orbitals
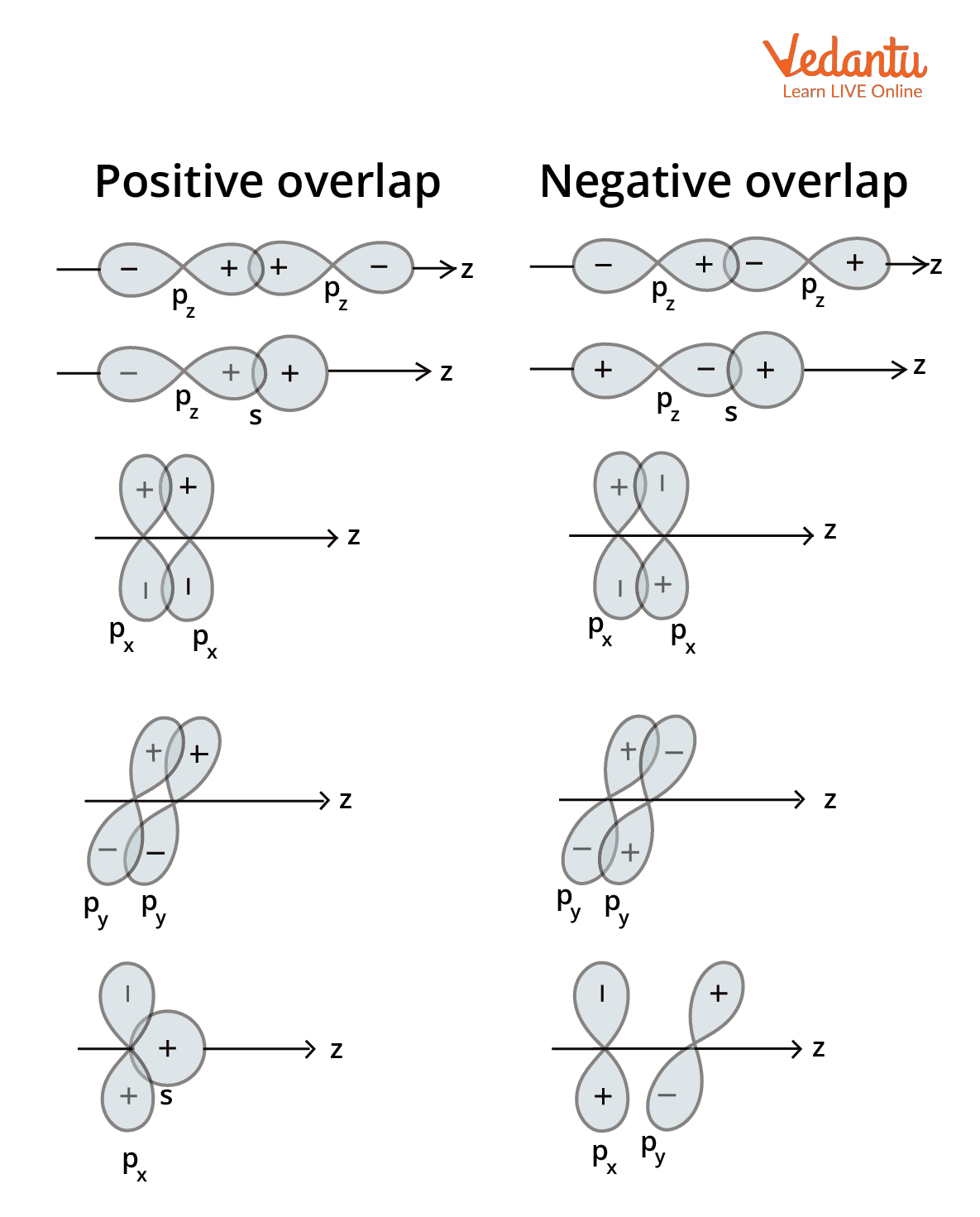
The atomic orbitals of 2 atoms overlap once they are nearer to one another. The overlapping of atomic orbitals can have positive, negative, or zero overlaps, relying upon these characteristics. The figure beneath shows the different configurations of the s and p-orbitals that lead to positive, negative, and zero overlaps.
Positive atomic orbital overlap: Whenever the two involved atomic orbitals phase is identical, positive overlap takes place. Bonds are created as a consequence of this overlap.
Negative atomic orbital overlap: Negative overlap occurs whenever the phases of the involved atomic orbitals oppose one another. Bond formation doesn't take place in this instance.
Zero overlaps of atomic orbital: Zero atomic orbital overlaps occur while two intriguing orbitals do not overlap with each other in an orbital.
The orbital overlap diagram is shown below.
Structure of Orbital Overlap
Orbital Overlap in Cumulene Compounds
The existence of 2 main carbon atoms carrying 2 double bonds accounts for the orbital overlap in cumulene compounds' rigidity. Due to the sp hybridisation of such carbon atoms, 2 π bonds—one to near each carbon atom—are formed. Cumulene molecules thus possess linear geometry. Hybridisation of cumulene contains 9 (σ) and 2 (π) bonds.
Key Features of Orbital Overlap
The phrase "atomic orbital overlap" is another name for orbital overlapping.
Linus Pauling highlighted the significance of orbital overlap while characterising the molecular bond angles found during experimentation.
The idea of orbital hybridisation also represents an additional development of orbital overlapping.
Orbital overlap refers to the methodology whereby a partial merger of orbitals creates a completely novel hybridised orbital. The overlapping regions of the orbitals are called pi (π) and sigma (σ).
Bond-forming orbitals must have the same orientation and mode in space.
The pair of atoms involved, their size, and valence electrons all play a role in determining the degree of overlap level. Higher levels of overlap result in the atoms forming firmer bonds with one another.
Conclusion
So, bond chemistry helps analyse the orbital overlap that happens when two atoms mingle with molecules. With the help of bond chemistry, orbital overlap theories VBT and MOT explain the atomic orbital overlap, while VBT specifically explains the orbital hybridisation concept where MOT falls short.
FAQs on Orbital Overlap Explained: Key Principles for Students
1. What exactly is orbital overlap in chemistry?
Orbital overlap is the concept used to explain how a covalent bond forms. It describes the process where atomic orbitals from two different atoms merge in the same region of space. This sharing of space allows a pair of electrons to be attracted to both nuclei, holding the atoms together and forming a stable chemical bond.
2. What are the main types of covalent bonds formed by orbital overlap?
There are two primary types of covalent bonds formed through orbital overlap:
- Sigma (σ) bonds: These are formed by the direct, head-on overlap of atomic orbitals along the internuclear axis (the imaginary line connecting the two nuclei). Sigma bonds are the strongest type of covalent bond.
- Pi (π) bonds: These are formed by the sideways or lateral overlap of p-orbitals above and below the internuclear axis. Pi bonds are generally weaker than sigma bonds and only form after a sigma bond is already present.
3. What are the three types of orbital overlap based on phase?
The effectiveness of orbital overlap depends on the phase (the sign of the orbital wave function) of the interacting orbitals:
- Positive Overlap: Occurs when two orbitals with the same phase sign (+ and + or - and -) interact. This leads to constructive interference and the formation of a stable bonding molecular orbital.
- Negative Overlap: Occurs when two orbitals with opposite phase signs (+ and -) interact. This leads to destructive interference and the formation of an unstable anti-bonding molecular orbital.
- Zero Overlap: Occurs when the orbitals have different symmetries and there is no net overlap. For example, an s-orbital attempting to overlap with a p-orbital sideways. No bond is formed in this case.
4. How is a pi (π) bond different from a sigma (σ) bond?
Sigma and pi bonds differ in several key ways:
- Formation: Sigma bonds are formed by head-on overlap, while pi bonds are formed by sideways overlap.
- Strength: Sigma bonds are stronger because the extent of overlap is greater.
- Rotation: Atoms can rotate freely around a sigma bond, but rotation is restricted around a pi bond.
- Occurrence: A single bond is always a sigma bond. A double bond consists of one sigma and one pi bond, and a triple bond has one sigma and two pi bonds.
5. What is a simple example of orbital overlap in a molecule?
A classic example is the formation of a hydrogen molecule (H₂). Each hydrogen atom has one electron in its spherical 1s orbital. When two hydrogen atoms approach each other, their 1s orbitals overlap to form a sigma (σ) bond. The two electrons are then shared in the region of overlap between the two nuclei, creating a stable H₂ molecule.
6. Why does orbital overlap lead to a more stable molecule?
Orbital overlap creates a region of high electron density between the two positively charged nuclei. This concentration of negative charge attracts both nuclei simultaneously, overcoming the repulsion between them. This process releases energy and lowers the overall potential energy of the system, resulting in a more stable arrangement than two separate atoms.
7. What is the difference between orbital overlap and hybridisation?
While related, they are distinct processes. Hybridisation is the mixing of atomic orbitals within a single atom to form a new set of identical hybrid orbitals (like sp³, sp², sp) *before* bonding occurs. Orbital overlap is the subsequent interaction between the orbitals (hybrid or atomic) of *two different atoms* to form the actual covalent bond.
8. Does the shape of an atomic orbital affect how it can overlap?
Yes, the shape and orientation of an orbital are crucial. For example:
- An s-orbital is spherical and non-directional, so it can only form sigma (σ) bonds through head-on overlap.
- A p-orbital is dumbbell-shaped and has direction. It can form a sigma bond if it overlaps head-on with another orbital, or it can form a pi (π) bond if it overlaps sideways with another p-orbital.
This directional nature determines the geometry and bonding capabilities of an atom.
9. How do we show orbital overlap in diagrams?
In chemistry diagrams, orbital overlap is shown by drawing the shapes of the interacting atomic orbitals (e.g., spheres for s-orbitals, dumbbells for p-orbitals) and showing them merging or intersecting. The phase of the orbital lobes is often indicated with shading or plus (+) and minus (-) signs to show how they interact to form a bond.
























