




Interesting Plutonium Facts
Plutonium is the 94th element in the periodic table, it is a radioactive element and is majorly synthetically made. Although scientists have discovered small amounts of naturally occurring plutonium created under incredibly rare geological conditions, plutonium is still regarded as a man-made material. It is mainly used in the nuclear industry like making nuclear weapons like atomic bombs because of its radioactivity. In this article we will state some interesting facts about this element and also talk about its symbol, electronic configuration, structure and later state some of its uses in today’s industries.
Plutonium on the Periodic Table
Plutonium (Pu), atomic number 94 in the actinide family of the periodic table, is a radioactive chemical element. Due to its employment as nuclear reactor fuel and a component of nuclear weapons, it is the most significant transuranium element.
Plutonium on Periodic Table
Characteristics of Plutonium
With an atomic number of 94, plutonium is the second transuranic element and a member of the actinide group on the periodic table.
Like neptunium, which was named after the planet Neptune, Plutonium is named after the planet Pluto. It is an actinide metal that seems silvery-grey; yet, when exposed to oxygen, it develops a dull covering.
There are six different allotropes and four oxidation states of this element. With nitrogen and hydrogen, it reacts.
When the metal is present in high concentrations, alpha decay causes the metal to warm up to the point where water can boil.
Plutonium is primarily produced by irradiating uranium in nuclear reactors, with uranium serving as the principal source of this element. Typically, nature does not contain plutonium.
Name: Plutonium
Symbol of Plutonium: Pu
Atomic Number: 94
Atomic Mass: (244.0) amu
Number of Protons/Electrons: 94

Plutonium characteristics
Specifications of Plutonium
With the majority of metals, plutonium forms intermediate compounds and alloys.
Plutonium and other transuranium elements like Neptunium, Americium, and Curium pose a radioactive risk and should be treated with caution.
At normal temperature, plutonium has a very high resistance. Even at low temperatures, it is high.
When melted, plutonium increases its density by 2.5%; however, when compared to other metals, it has a relatively high surface tension and viscosity.
Uses of Plutonium
Due to its radioactive nature, plutonium isn't particularly useful in any application.
The use of this element as an energy source is one of its primary uses. The early atomic bombs included it.
The element is employed in nuclear weapons because it is a crucial fissile component, is more readily available, and fissions more easily than other elements.
Plutonium-239, one of its isotopes, is a component of modern nuclear bombs.

Plutonium-238 Used in NASA
Facts About Plutonium
Plutonium Element: It is an element which belongs to group 20 and period 7 in the periodic table.
Plutonium Electrons: Plutonium has in total 94 electrons.
Plutonium Atomic Structure:
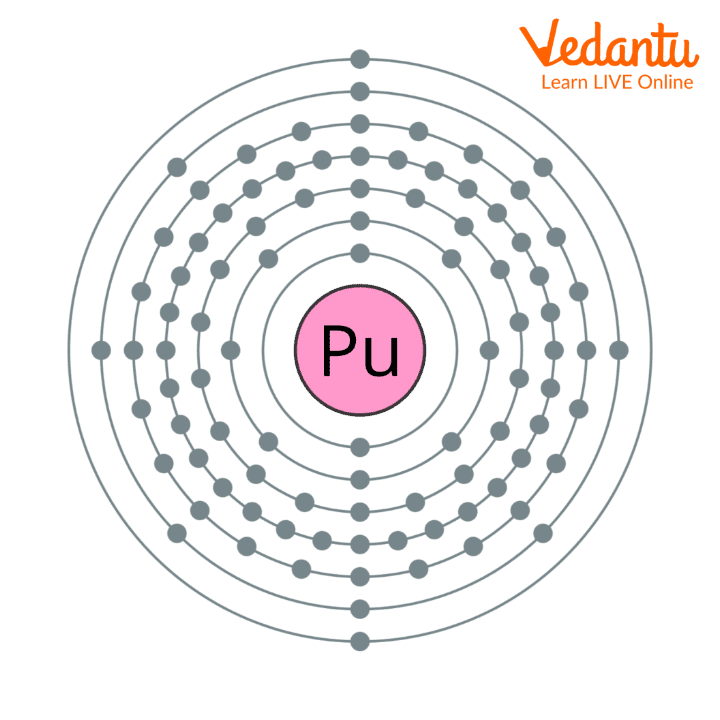
Plutonium Atomic Structure
Plutonium Symbol:

Plutonium Symbol.
A silver metal with radioactivity, plutonium may be employed for both creation and destruction. Even though it was once employed for destruction, today the element is mostly used for energy creation all over the world.
In nature, plutonium is seldom encountered. Naturally occurring uranium ores include trace amounts of plutonium.
Sample Questions
1. What is plutonium's electronic configuration?
Ans: Pu- atomic number : 94
Electrical configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2
2. Plutonium has a half-life of 24000 years before it decays. The portion of plutonium left after 72000 years of storage is
⅛
⅓
¼
½0
Learning by Doing
1. Plutonium is found in ______.
Nature.
Space.
Uranium ores.
The ocean.
Ans: (C)
In nature, plutonium is seldom encountered. Naturally occurring uranium ores include trace amounts of plutonium.
2. What is the shelf life of plutonium?
60 years
82 million years
1000 years
314159265 days
Ans: (B)
The longest-lasting isotope of plutonium is plutonium-244. Its half-life is approximately 82 million years.
Summary
Plutonium is the 94th element in the periodic table and is one of the rare elements. It has three stable isotopes and is mainly used in the nuclear industry since it is a radioactive element.
A crucial component in the creation of nuclear power is plutonium. Numerous of the earliest atomic bombs contained plutonium, which is being utilized in nuclear weapons today. An explosion equal to more than 10,000 tonnes of chemical explosive is produced by the entire detonation of one kilogram of plutonium.
FAQs on Facts About Plutonium
1. What is Plutonium and where is it on the periodic table?
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. On the periodic table, it is classified as an actinide metal, positioned in the f-block in period 7.
2. How is plutonium produced?
Plutonium is primarily a synthetic element. It is most commonly produced in nuclear reactors by bombarding uranium-238 with neutrons. While trace amounts can occur naturally in uranium ores under extremely rare conditions, it is largely considered a man-made material.
3. What are the main uses of plutonium in modern technology?
Plutonium has several key applications based on its specific isotope:
- Plutonium-239: As a primary fissile component, it is crucial for creating nuclear weapons and is also used as a fuel in some nuclear power reactors.
- Plutonium-238: Due to the significant heat produced during its decay, it is used as a long-lasting power source for spacecraft and rovers on NASA missions, as well as in radioisotope thermoelectric generators (RTGs).
4. Is plutonium a metal?
Yes, plutonium is a metal. It is a dense, silvery-grey actinide metal that quickly develops a dull, tarnished coating when it is exposed to oxygen in the air. However, unlike most metals, it is a poor conductor of heat and electricity.
5. What is the electronic configuration of Plutonium?
The electronic configuration for Plutonium (Pu), which has an atomic number of 94, is [Rn] 5f⁶ 7s². This configuration places it within the actinide series of the periodic table, which is a key topic in advanced chemistry.
6. Why is plutonium considered so dangerous?
Plutonium's danger stems from its intense radioactivity. It is primarily an alpha emitter, and if inhaled or ingested, these alpha particles can cause severe damage to internal tissues and organs, leading to cancer. Additionally, like other heavy metals, it is chemically toxic to the body.
7. What makes plutonium's physical properties unusual compared to other metals?
Plutonium is unique due to several properties not typically seen in metals. It has a relatively low melting point but a very high boiling point. It is a poor conductor of heat and electricity. Most notably, it exists in six different allotropes at standard pressure, each with a different crystal structure and density, which change with temperature.
8. How does plutonium generate heat on its own?
Plutonium generates its own heat through the process of radioactive decay, specifically alpha decay. The unstable nucleus of a plutonium atom releases an alpha particle, converting some of its mass into energy. This continuous release of energy manifests as heat, making a sufficiently large piece of plutonium warm to the touch and capable of boiling water.
9. Why is the isotope Plutonium-239 more important for nuclear applications than other isotopes?
Plutonium-239 is particularly important because it is fissile. This means its nucleus can be split by a slow-moving neutron, releasing a tremendous amount of energy and more neutrons. This ability to create a self-sustaining nuclear chain reaction is what makes it an essential component for both nuclear power generation and nuclear weapons.
10. What does it mean for plutonium to have multiple allotropes?
Having multiple allotropes means that plutonium can exist in different solid structural forms, even though it is the same element. For plutonium, this is significant because each of its six allotropes has different physical properties, such as density and thermal expansion. This complex behaviour, which is sensitive to changes in temperature, pressure, and impurities, makes machining and working with plutonium very challenging.









