




Why is Helium Unique? Discover Its Role in Science & Daily Life
Do you remember the last time you had one of those super light balloons stuck to the roof? Ever wondered what made the balloon so magical? Or if you are into celebrity interviews these days, you might have noticed these celebrities inhaling from a balloon to sound like a chipmunk! If you are wondering what is common between all of the things mentioned above, it is helium. Let us get to know this new chemical friend to see things around us with a better understanding.

Helium Balloons
What is Helium?
Helium is the lightest gas known to man (well technically, lightest after hydrogen). It is known to be a part of this family called the ‘Noble gases’ due to their ‘noble’ property to stay calm and unreactive during chemical reactions. It is not very easily found on our planet, but guess what? The super giant sun is a boiling magma of Helium (We’ll jump into the details of that in a short while). In other words, not widely available on earth but is present in abundant quantities in the universe.
Who Found Helium?
Helium has an interesting story of discovery. Mr. Pierre Janssen (A French astronomer, see astronomer contributing towards chemistry!) and Norman Lockyer (An English Scientist and astronomer) decided to turn their telescopes towards the sun on the day of the solar eclipse. In doing so, they observed a peculiar bright-yellow line among the spectrum (a band of colours, basically) produced by the sun.
Now that the observation was done, they had to think about it as this specific line matched nothing of what they have ever come across (this was back in 1868), so Mr. Norman finally concluded that the phenomenon was due to a new element whose existence on earth was still unknown but found in the sun. The best part of the story is the name of the element. “Helium” is derived from the ancient Greek word “Helios” meaning sun referring to its presence in the sun.

Norman Lockyer
Pierre Janssen
Where is Helium Found?
Following the discovery in 1868, we’ve come a long way in terms of using this element for our betterment in various aspects. Our dependency on this helium has tremendously increased. To meet these needs, how is this element obtained? A few sources of helium are listed below:
From the upper atmosphere of earth where it is present due to its light-weight. It is rarely found on earth as it easily escapes into our space (just like the balloon we earlier mentioned!)
The decay of heavy elements (Uranium) produces alpha particles. Alpha particles are Helium nuclei with a positive charge.
Extraction from natural gases (gases that catch fire easily and are hence used as fuels) called fractional distillation (the mixture of gas is boiled and separated over various temperatures.)
Natural helium gas deposits constantly release helium gas.
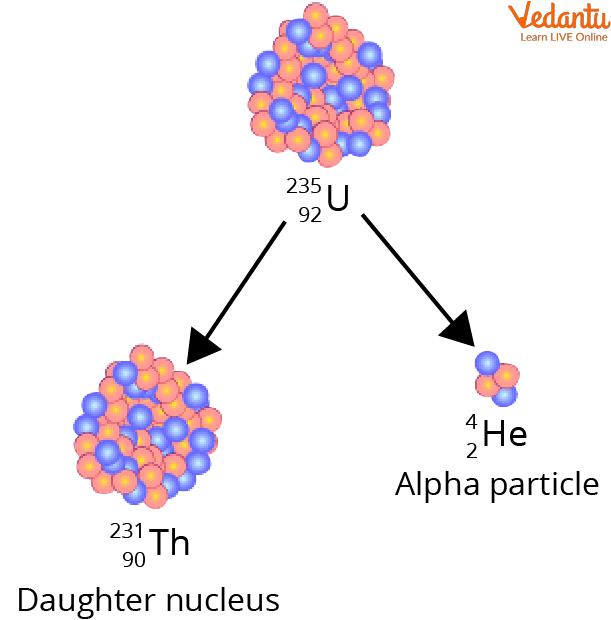
Alpha Decay of Uranium to Produce Helium
What are the Properties of Helium?
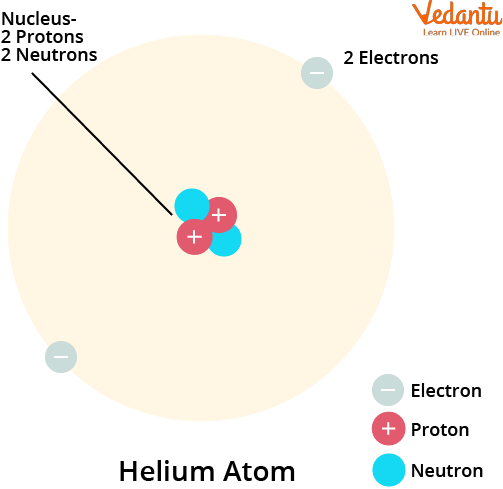
Starting from the very basics, Helium is denoted by the chemical symbol He.
It consists of a single atom (known as monatomic).
It is odourless, colourless, and tasteless gas.
It has low weight (lighter than air).
Very unreactive but reacts with other elements when made to react under drastic conditions.
It is very stable and highly insoluble.
Produces a cooling effect with hydrogen due to concentration difference.
Monatomic Helium
What are the Uses of Helium?
Despite its limited sources, helium is extensively used by various industries as fuel, as a chemical medium, as a source of transport, and so on. A short list of its uses is given below:
As a source of transport due to its light-weight and non-inflammable property (it does not catch fire if it comes in contact with a flame).
Producing a cool environment for superconducting magnets used for MRI scans.
Used in Cryogenics (Preserving substances by freezing them under extremely low temperatures).
As a constituent in welding flame.
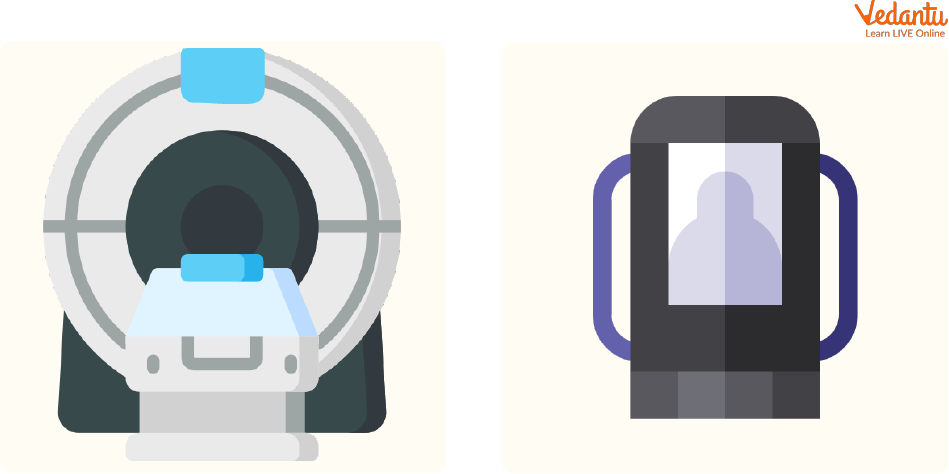
Uses of Helium in MRI and Cryogenics
Summary
Helium is the lightest gas known to man after hydrogen. Known as the ‘noble gas’ due to its unreactive nature it does not form compounds with other elements under normal conditions. Properties of helium are: it is a monatomic, tasteless, colourless, odourless gas having low solubility in water. Often obtained from the upper atmosphere due to its lightweight and density, Helium denoted as He is a gas of commercial importance. It is used in cryogenics, as fuel, in MRI scans and also to provide an unreactive reaction medium in the laboratory.
FAQs on Helium Gas: Definition, Properties & Real-Life Uses
1. What is helium and what are its key properties?
Helium is a chemical element and the second lightest element in the universe. Its key properties include being a colourless, odourless, and tasteless gas. As a noble gas, it is chemically very stable and inert, meaning it does not easily react with other elements. It also has the lowest boiling and melting points of all the elements.
2. What is the chemical symbol and atomic structure of helium?
The chemical symbol for helium is He. Its atomic number is 2, which means a neutral helium atom contains two protons and two electrons. Its electron configuration is 1s², representing a completely filled first electron shell. This stable configuration is the primary reason for its chemical inertness.
3. What are the most important uses of helium gas in science and industry?
Due to its unique properties, helium has several critical applications. The most important uses include:
- Cryogenics: As a liquid, it is used as a super-coolant for the powerful magnets in MRI scanners and NMR spectrometers.
- Lifting Gas: It is used to fill large weather balloons and airships because it is much lighter than air and, crucially, non-flammable.
- Welding: It provides an inert protective atmosphere for high-temperature arc welding of certain metals.
- Leak Detection: Its small atomic size allows it to be used to detect tiny leaks in high-vacuum systems and other sealed containers.
4. Why is helium used in breathing mixtures for deep-sea divers?
Deep-sea divers use a mixture of oxygen and helium called Heliox to prevent a dangerous condition known as decompression sickness or "the bends." Helium is used because it is significantly less soluble in blood than nitrogen (which makes up about 78% of the air we breathe). This prevents gas bubbles from forming in a diver's bloodstream during their ascent to the surface, which can cause extreme pain and tissue damage.
5. Why is helium considered a rare and non-renewable resource on Earth?
Helium is rare on Earth primarily because it is extremely light. Its atoms are not heavy enough to be held by Earth's gravity and they gradually escape into space. On Earth, it is not manufactured but is generated very slowly from the radioactive decay of heavy elements like uranium and thorium trapped in the crust. Because this natural process is incredibly slow and the gas escapes our atmosphere, the helium we extract is a finite, non-renewable resource.
6. Why is helium preferred over hydrogen for filling party and weather balloons?
Although hydrogen is lighter and provides slightly more lift, it is highly flammable and can form explosive mixtures with air. The Hindenburg airship disaster is a famous historical example of this danger. Helium, on the other hand, is a noble gas, which means it is completely inert and non-flammable. This inherent safety makes it the preferred choice for any application where the risk of fire or explosion is a concern, such as in party balloons and public events.
7. How does helium's electron configuration explain its unique properties like inertness and its extremely low boiling point?
Helium's properties are a direct result of its simple and stable electron configuration, 1s².
- Inertness: Its outermost electron shell is completely filled with two electrons. This is a very stable arrangement, making helium extremely reluctant to gain, lose, or share electrons to form chemical bonds. This stability is why it is a noble gas.
- Low Boiling Point: As a small atom with only two electrons, the intermolecular forces of attraction between helium atoms (van der Waals forces) are exceptionally weak. Consequently, very little thermal energy is required to overcome these forces and allow the substance to boil, giving it the lowest boiling point of any element.









