




Why Understanding the Periodic Table Matters in Science
A tabular representation of the chemical elements is known as the periodic table, or the periodic table of the (chemical) elements. It is commonly regarded as an icon of Chemistry and is used frequently in Physics, Chemistry, and other sciences. It is a visual representation of the periodic law, which states the atomic number-dependent regularity of the properties of chemical elements.
The table is divided into four blocks that are roughly rectangular in shape. The table's columns are referred to as groups and its rows are known as periods. The chemical properties of elements in the same column group of the periodic table are similar.
Trends can be seen throughout the periodic table, with metallic characteristics (surrendering electrons to other atoms) increasing in the opposite direction from left to right across a period and from down to up across a group. The basic reason for these trends is atoms' electron configurations.
What is the Periodic Table?
The elements are listed in the Periodic Table. Elements are listed in the table based on how their atoms are arranged. This includes the number of protons and electrons in their outer shell. The elements are arranged from left to right and top to bottom according to their atomic number, or the number of protons in each atom.
Dmitri Mendeleev, a Russian chemist, put forth the periodic table in 1869. Before many of the elements were actually discovered, Mendeleev was able to predict their characteristics using the table.
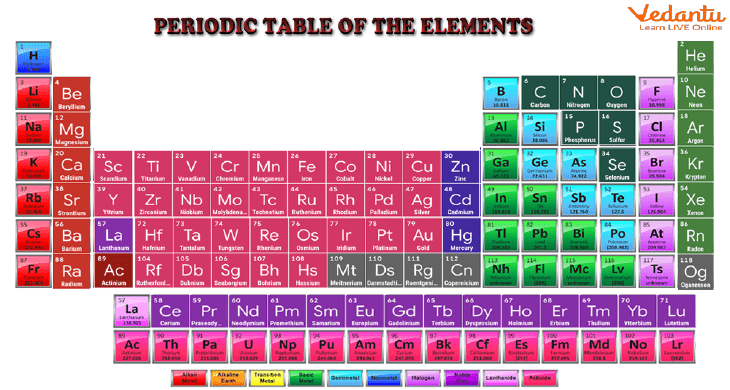
Periodic Table Examples of Elements for Kids
Why is it Called the Periodic Table?
The word "periodic" refers to how the elements are arranged in cycles or periods. According to their atomic number, elements are arranged from left to right in rows (the number of protons in their nucleus). To arrange elements on the same columns that have the same valency, some columns are skipped. The elements in the columns have similar characteristics when they are arranged in this manner.
The table's horizontal rows are separated by periods. There are a total of seven (or eight) periods. The first is brief and contains only hydrogen and helium. 32 elements make up the sixth period. The leftmost element in each period has one electron in its outer shell, while the rightmost element has a full outer shell.
Groups
The noble or inert gases are an example of a group. The periodic table's last or eighth column corresponds to all of these elements. Since each of them has a full outer shell of electrons, they are all very stable (they tend not to react with other elements). Another example is the alignment of the alkali metals, which are all visible on the left-most column. They are all extremely reactive and have just one electron in their outer shell, which makes them all quite similar. The table below contains a list of every group.
When working with elements, chemists find it helpful when similar elements are lined up and grouped together. They are able to comprehend and predict a given element's potential behaviour or reaction.
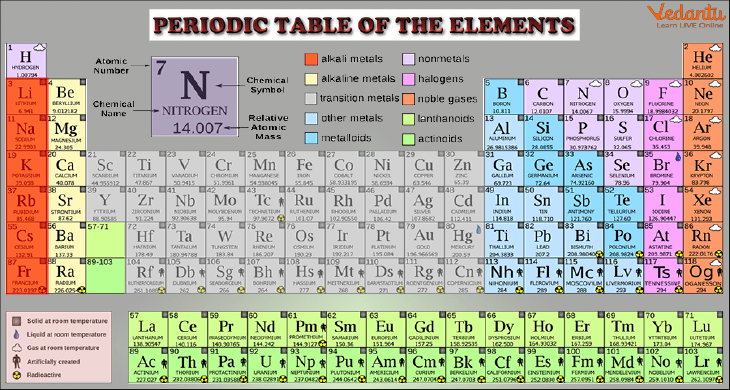
Periodic Table with Detailed Information
Element Abbreviations
In the periodic table, each element has a unique name and abbreviation. Some of the abbreviations, like H for hydrogen, are simple to recall. Others, like Fe for iron or Au for gold, are a little tougher. The Latin word for gold, aurum, is where the letter "Au" in gold is derived.
Interesting Facts About the Periodic Table
The ability of carbon to form up to 10 million different compounds makes it special. Life requires carbon in order to exist.
The most uncommon element on earth is francium. Only a few ounces of it are most likely present on earth at any given time.
Only the letter J is absent from the periodic table.
The Latin word for silver, Argentum, which has the symbol Ag, serves as the inspiration for the name of the nation Argentina.
Helium is present on Earth, but it was first identified by studying the Sun.
Summary
The periodic table has 18 vertical columns. A group is what we call each column. Because each member of a group of elements has the same number of outer electrons, they all share similar chemical and physical characteristics. Periods: The elements in the periodic table are arranged in a grid of rows.
FAQs on Essential Periodic Table Facts Every Student Should Know
1. What is Be in the periodic table and what is the heaviest element?
Beryllium (Be), formerly known as glucinium (up until 1957), is a chemical element that is the lightest of the alkaline-earth metals in Group 2 (IIa) of the periodic table. It is used in metallurgy as a hardening agent as well as in numerous nuclear and outer space applications.
Oganesson, which bears the name of the Russian physicist Yuri Oganessian (SN: 1/21/17, p. 16), is currently the heaviest element in the periodic table, with a massive atomic mass of about 300. The synthetic element has only ever been produced in very small numbers, and each atom only managed to survive for a fraction of a millisecond.
2. What is the periodic table of elements and what are elements of the family?
All discovered chemical elements are arranged in rows (referred to as periods) and columns (referred to as groups) in the periodic table of chemical elements, also referred to as the periodic table, in increasing order of atomic number.
The same number of valence electrons is a characteristic of element families. The majority of element families are represented by a single column in the periodic table, but the transition elements and the elements that are listed below the main body of the table each have their own set of columns.









