




Key Differences and Properties of Solids, Liquids, and Gases
Water can exist as ice, liquid, and even vapours which means it can exist in variable states. Have you ever wondered how some objects are rigid whereas others are easily changing their shape? This is all due to the state in which a matter exists. Variable states provide various properties to any matter.

States of the Matter
States of matter means different or distinct characteristics and properties a matter can exhibit. It is also known as the phases of matter. In general, there are three states of matter, i.e., solid, liquid, and gas. Every state of matter has unique properties in very different environmental conditions which form the basis of the classification. Every state of matter is exhibited around us from a solid sofa to liquid cold drinks and from water to gaseous air that we breathe. Every state of matter has an essential part in the environment and our day-to-day life.
Solids, Liquids, and Gases
Solids
Solids are tough and have a rigid shape which implies that they cannot be moulded into other shapes easily.
They occupy a definite space.
Have a fixed shape and volume.
Have high intermolecular attraction and less or no space between consecutive molecules which provides them rigidity.
Do not tend to flow like liquids and cannot be easily compressed.
Example - wood, ice, brick, stone.

Examples of Solids
Liquids
Liquids have fixed volume but do not have a fixed shape. They occupy space and change their shape according to the container they are placed in.
They have a higher density, and more intermolecular space between them, unlike solids.
The liquid particles can flow easily and freely.
Can be compressed to a certain extent.
Examples - blood, water, milk, coffee.
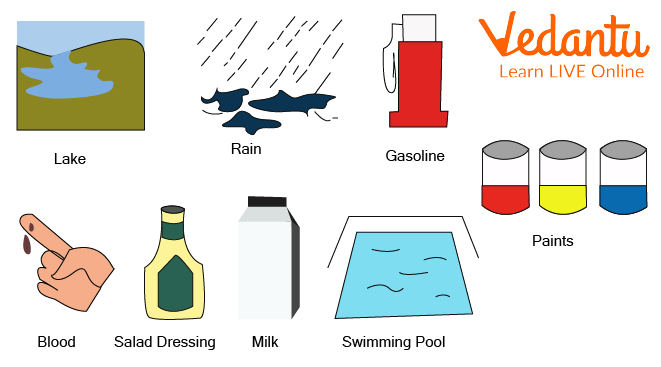
Examples of Liquids
Gas
They neither have a fixed volume nor a fixed shape.
They have much less intermolecular space between them compared to solids as well as liquids.
Gases are easily liquified or compressed.
They fill the space complete in which they are kept.
Particles have very little interaction and are randomly moving.
Examples - oxygen, carbon dioxide, neon, etc.
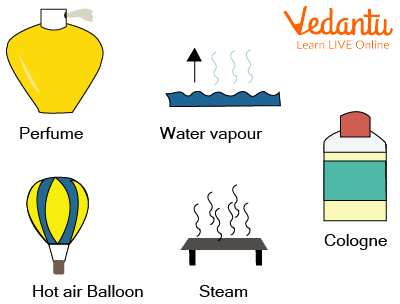
Examples of Gaseous State
Differences between Different States of Matter
Difference between Solids and Liquids
Summary
There are three states of matter that exist in distinct physical forms under different environmental conditions. These matters are usually classified according to the basis of intermolecular forces and space between the molecules. These states can be changed into each other by various processes.
FAQs on Solids, Liquids and Gases Explained
1. What are solids, liquids, and gases?
Solids, liquids, and gases are the three main states of matter that we encounter every day. A simple way to understand them is by their properties:
Solids, like a book or a rock, have a fixed shape and take up a fixed amount of space (volume).
Liquids, like water or milk, take the shape of their container but have a fixed volume.
Gases, like the air we breathe, have no fixed shape or volume and spread out to fill any space they are in.
2. What are some common examples of solids, liquids, and gases in our daily life?
We are surrounded by all three states of matter. Here are some easy examples:
Examples of Solids: A table, a pencil, an apple, and ice cubes.
Examples of Liquids: Drinking water, juice, milk, and rain.
Examples of Gases: The air (a mix of gases like oxygen), steam from a kettle, and the helium inside a balloon.
3. How do the properties of solids, liquids, and gases compare?
The main differences between solids, liquids, and gases are based on their shape, volume, and how their particles are arranged.
Solids: Have a definite shape and definite volume because their particles are packed tightly together and can only vibrate.
Liquids: Have no definite shape but a definite volume. Their particles are close but can slide past one another, allowing them to flow.
Gases: Have no definite shape and no definite volume. Their particles are far apart and move freely and quickly in all directions.
4. What happens to water when you heat it or freeze it?
Water is a perfect example of how matter can change its state. When you take heat away from water by freezing it (at 0°C), it turns from a liquid into a solid called ice. When you add heat to water by boiling it (at 100°C), it turns from a liquid into a gas called steam or water vapour. This shows that the same substance can exist in all three states.
5. Why do gases fill any container they are in, but liquids only take the shape of the bottom part?
This difference is all about the energy and movement of their particles. The particles in a gas have lots of energy and are very far apart, allowing them to move around freely and rapidly to fill the entire container. In a liquid, the particles are closer together and have less energy. They are attracted to each other, so they can't escape and spread out; instead, they slide over one another to take the shape of the container they are in, starting from the bottom.
6. Is a rubber band considered a solid even though it can change its shape?
Yes, a rubber band is considered a solid. This is a common point of confusion. Although you can stretch it to temporarily change its shape, it always returns to its original shape when you release the force. This property of having a definite shape that it tries to maintain is a key characteristic of solids. It does not flow like a liquid or expand to fill a room like a gas.
7. Why is ice cream often described as a mix of a solid, liquid, and gas?
Ice cream is a fascinating example because it's not just one state of matter. It is a mixture that contains all three:
Solid: Tiny ice crystals give it its firmness and coldness.
Liquid: A thick, sugary syrup component that doesn't completely freeze, keeping it soft enough to scoop.
Gas: Small air bubbles are whipped into the mixture to make it light and creamy.
So, when you eat ice cream, you are experiencing a combination of all three fundamental states of matter!
8. If we can't see most gases, why are they so important for life on Earth?
Even though most gases are invisible, they are essential for survival. The most important gas, oxygen, is in the air we breathe and is needed by almost all living creatures to produce energy. Another gas, carbon dioxide, is used by plants to make their food through photosynthesis. The atmosphere, which is a blanket of gases around the Earth, also protects us from the sun's harmful rays and keeps our planet warm enough for life to exist.
9. Are there other states of matter besides solid, liquid, and gas?
Yes, while solid, liquid, and gas are the three states we see in our normal lives, scientists have identified other states of matter that exist under extreme conditions. The two most well-known are:
Plasma: A super-heated, energised gas that is found in stars like our Sun, and in lightning.
Bose-Einstein Condensate (BEC): A state that occurs at extremely cold temperatures, where atoms clump together and act as a single entity.









