




What are Beta Rays?
Beta particles are high energy, fast electrons (-) or positrons (+) released from the nucleus by some radioactive elements during a kind of radioactive decay known as beta-decay. Normally, beta-decay happens in nucleus with too many neutrons to achieve stability.
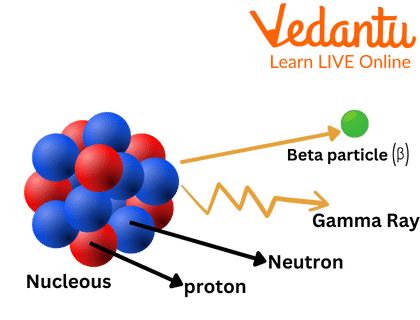
Beta Particle
Uses of Beta Rays
These particles have certain helpful qualities due to their moderate penetrating power. As a result, beta particles are used in a wide variety of applications. Tracers and material thickness monitoring are both done with beta radiation.
Tracers are radioactive substances that doctors may use for medical imaging. Certain chemicals concentrate in certain injured or diseased regions of the body, and radiation concentrates alongside them. Radiation detectors positioned outside the body detect the radiation emitted and create an image of the inside of the body using computers.
Radiation is used to measure the thickness of materials like paper, plastic, and aluminium. The more radiation that is absorbed, the less radiation that reaches the detector.
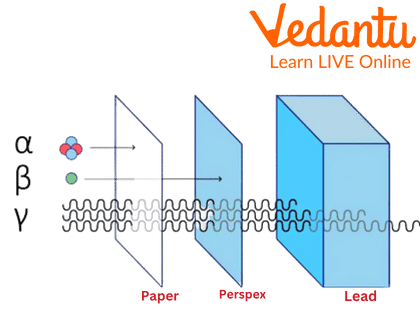
Uses of Beta Gamma and Alpha Rays and Its Effectiveness
Application of Beta Rays
Beta particles can be used to treat diseases such as eye and bone cancer, as well as tracers. The most frequent substance utilised to create beta particles is strontium-90.
Beta particles are also employed in quality control to measure the thickness of an item, such as paper, as it passes through a roller system. While travelling through the product, some of the beta radiation is absorbed. If the product is too thick or too thin, a different amount of radiation will be absorbed.
A computer programme that monitors the quality of the created paper will then move the rollers to change the end product's thickness.
Uses of Beta Radiation in Industry
Positron emission tomography also makes use of the beta-plus (or positron) decay of a radioactive tracer (PET scan).
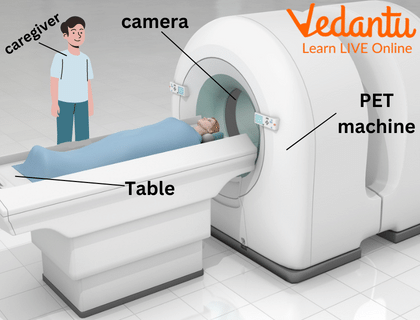
PET Scan
In industry, radiation is used in detectors to monitor and manage the thickness of materials such as paper, plastic, and aluminium. The more radiation that is absorbed, the less radiation that reaches the detector. It then sends signals to the machinery that regulates the material's thickness.
Doctors also use radioactive substances called "tracers" for imaging purposes. Tracers and material thickness monitoring are both done with beta radiation.
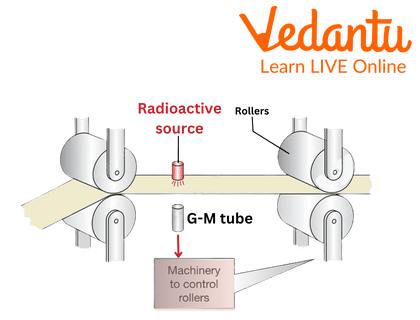
Use of Beta Radiation in Industry
Here, the radioactive source is the beta rays
Uses of Beta Radiation in Medicine
Beta particles can be used to treat diseases such as eye and bone cancer, as well as tracers.
Beta-emitting radiopharmaceuticals (substances used to identify or treat certain medical issues or disorders), such as radioimmunotherapy and bone-seeking radiopharmaceutical therapy, are finding broader applications in cancer treatment. Beta-emitting radioisotopes have also been widely employed in vascular brachytherapy (the use of radioactive implants to treat cancer, particularly prostate cancer, directly within the tissue) and other forms of brachytherapy.
Beta Radiation Used in Daily Life
The manufacturing industries use beta decay of elements for a variety of applications.
One of the most visible applications of beta decay in real life can be found in medicine. A variety of diagnostic and therapeutic devices used in hospitals and biology laboratories rely on the beta decay of radioactive elements such as uranium, thorium, and others.
The use of beta decay in the decomposition of a deceased organism's body is plainly recognised, as the carbon element, C-14, begins to decay and is changed into nitrogen, N-14.
Beta-decay is frequently employed in surgical procedures. For example, in the case of glaucoma surgery, beta decay or beta radiation are frequently preferred.
Surgery

Decomposition of the Body
Solved Examples
1. What effect do beta rays have on humans?
Ans: Beta particles can enter the skin and cause radiation damage, such as skin burns. Beta emitters, like alpha emitters, are most dangerous when breathed, eaten, or absorbed into the bloodstream through wounds.
2. What is the method for detecting beta radiation?
Ans: Scintillation counters are capable of detecting alpha, beta, and gamma radiation, as well as neutrons.
3. Where can you find beta particles?
Ans: The nucleus of the atom generates beta radiation. A neutron within the nucleus is transformed into a proton and an electron, and the electron is expelled in beta emission.
Conclusion
Although many investigations have been conducted without the use of beta rays, a few categories are required in the medical area, industry, cancer therapy, bone and skin disorders, and so on. These radiation existed many years ago, but there were no scholars who practically brought them, and they were used, but not in the way that they are currently employed. Because of the existence of these rays, technology is progressing from complicated to simple. These may be used in different ways in the near future.
FAQs on Uses of Beta Rays
1. What is a beta ray and how is it produced?
A beta ray is a stream of high-energy, high-speed electrons or positrons known as beta particles (β). These particles are emitted from the nucleus of a radioactive atom during a process called beta decay. This decay occurs when an unstable nucleus transforms to a more stable state by converting a neutron into a proton (emitting an electron) or a proton into a neutron (emitting a positron).
2. What are the main applications of beta rays in industry and science?
Beta rays have several important applications due to their moderate penetrating power. Key uses include:
- Thickness Gauging: In manufacturing, beta emitters are used to measure the thickness of materials like paper, plastic sheets, and aluminium foil without physical contact.
- Radioactive Tracers: Isotopes that emit beta particles, such as Carbon-14, are used as tracers to monitor complex chemical and biological processes.
- Radiocarbon Dating: The decay of Carbon-14 is a fundamental method used in archaeology to determine the age of organic materials.
- Quality Control: They are used in industrial processes to detect leaks in pipes and to ensure the correct filling level in containers.
3. How are beta rays used in the field of medicine?
In medicine, beta radiation is crucial for both diagnosis and therapy. Beta-emitting radioisotopes are used in brachytherapy, where a radioactive source is placed inside or next to the area requiring treatment, such as a cancerous tumour. This allows for a high dose of radiation to be delivered directly to the target cells while minimising damage to surrounding healthy tissue. Additionally, isotopes like Technetium-99m are used in imaging techniques to study organ function.
4. What are the key properties of beta particles?
Beta particles have distinct properties that determine their behaviour and applications:
- Charge: A beta particle can have a negative charge of -1 (electron) or a positive charge of +1 (positron).
- Mass: They have a very small mass, identical to that of an electron (approximately 1/1836th of a proton's mass).
- Velocity: Beta particles are emitted with a wide range of energies and can travel at speeds up to 99% of the speed of light.
- Penetrating Power: They are more penetrating than alpha particles but less penetrating than gamma rays. They can be stopped by a thin sheet of aluminium or a few millimetres of plastic.
5. How do the two types of beta decay, β⁻ and β⁺, differ from each other?
The two types of beta decay involve different transformations within the atomic nucleus. In beta-minus (β⁻) decay, a neutron is converted into a proton, and an electron (the beta particle) and an antineutrino are emitted. This increases the atomic number by one. In contrast, beta-plus (β⁺) decay involves a proton being converted into a neutron, with the emission of a positron (the beta particle) and a neutrino. This process decreases the atomic number by one.
6. Why are beta rays more penetrating than alpha particles but less than gamma rays?
The penetrating power of radiation is determined by its mass, charge, and energy. Alpha particles are large (two protons and two neutrons) and have a +2 charge, causing them to interact strongly with matter and lose energy quickly, stopping them within a few centimetres of air. Gamma rays are high-energy photons with no mass or charge, so they interact less frequently with matter and can penetrate very deeply. Beta particles are in the middle; their small mass and single charge lead to fewer interactions than alpha particles but more than gamma rays, giving them moderate penetrating ability.
7. How does a beta radiation-based thickness gauge work?
A thickness gauge works on the principle of radiation absorption. A constant source of beta radiation is placed on one side of the material being measured (e.g., a roll of paper), and a detector is placed on the opposite side. The amount of radiation that reaches the detector is inversely proportional to the thickness and density of the material. If the material becomes too thick, it absorbs more radiation, and the detector reading drops. This feedback is used in real-time to control the manufacturing process and maintain a consistent thickness.
8. What are some common examples of beta-emitting isotopes?
Several widely known isotopes undergo beta decay and are used in various applications. Common examples include Tritium (Hydrogen-3), used in self-illuminating signs; Carbon-14, used for radiocarbon dating; Strontium-90, used in industrial gauges and for medical treatments; and Technetium-99m, one of the most common radioactive tracers in medical diagnostics.









