




How Distillation Works: Important Processes and Practical Benefits
From the fuel that runs our cars to the water we drink are some of the distillation examples in everyday life; distillation is the source of many of the items we use practically and every day. There are several uses for distilled water, including in low-volume humidifiers and lead-acid batteries. With this technique, many fermented items, including alcoholic beverages, are cleansed. Cooling distillation is a method for dividing air into nitrogen, oxygen, and argon.
Uses of Distillation
There are various practical uses of distillation in daily life, as follows:
Purification of Water - Natural water comes with many minerals and contaminants, many of which can be eliminated through distillation. Distilled water is frequently used where the presence of minerals could diminish the efficiency of specific equipment, such as steam irons or cigar humidors. Some people prefer the flavour of distilled water or choose to stay away from the minerals in tap water.
Alcoholic Drinks - Whiskey, rum, and brandy are just a few alcoholic beverages made through distillation. A diluted form of ethyl alcohol is created during fruit and plant components fermentation. During the distillation process, a wide range of other components are also gathered, including water, esters, and various forms of alcohol.
Petroleum Products - Oil refinery waste can be used to make various items. Oil is refined into distinct materials utilising a procedure known as fractional distillation since each of these products has a different boiling point. These consist of fuel oil, diesel fuel, and lubricating oil.
Perfume - Making perfume was one of the first applications of distillation, which started approximately 3500 B.C. Essential oils, which can be obtained through distillation, contain the fragrance of several plants and herbs.
Food Flavourings - Natural food flavourings are also made using steam distillation. Citrus oils and liquid extracts of different herbs and spices are the most popular.
Scientific Applications - A real-world application for distillation is in the lab. Although the products of this kind of distillation might not end up in our homes, the technique is frequently employed in chemical and pharmaceutical research and quality assurance testing for numerous consumer goods.
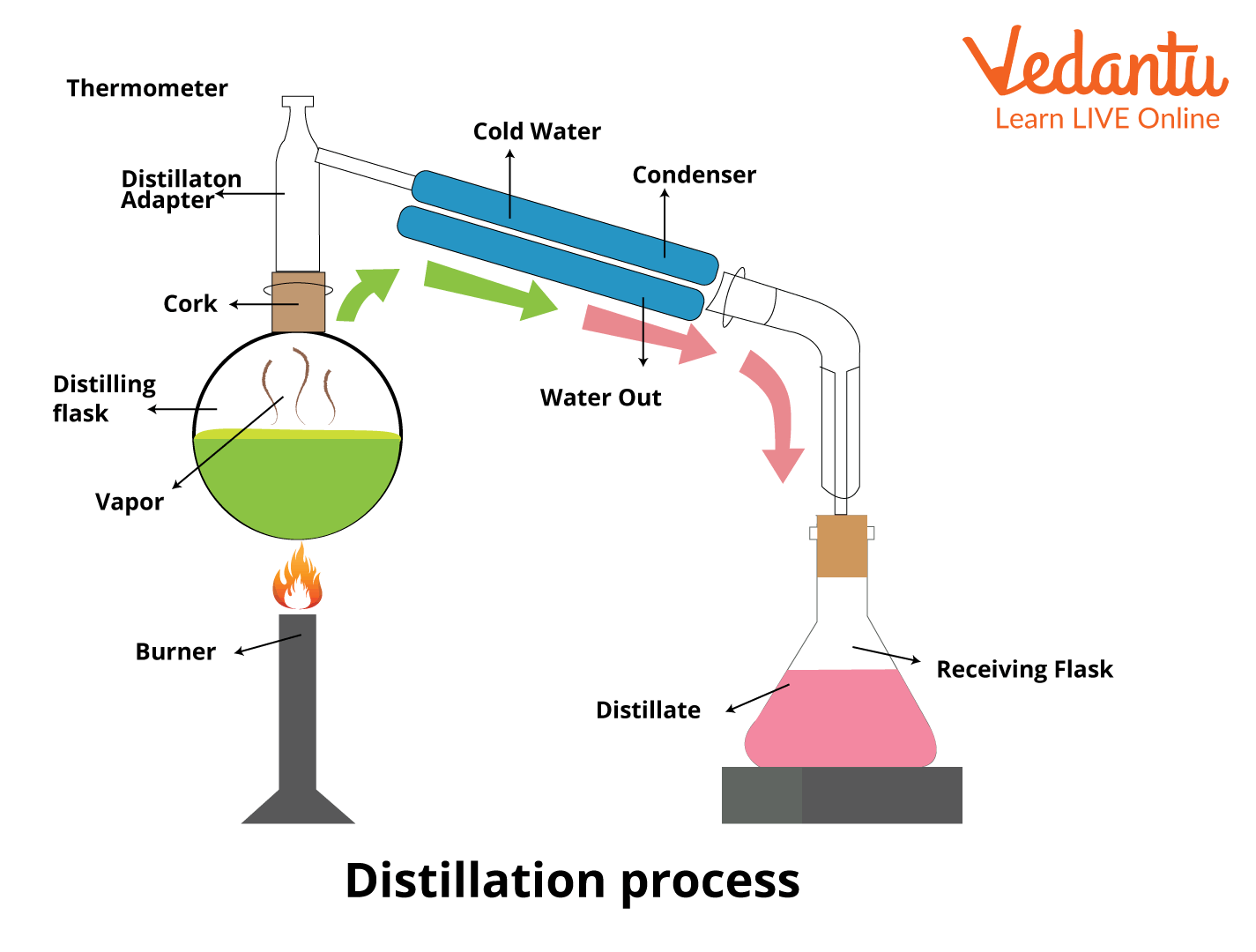
Distillation Process
What is Distillation?
Distillation is selectively boiling a component in a liquid mixture and then condensing it. It is a method of separation that can be applied to either get more of one particular component out of a mixture or to separate it almost completely.
By pushing one of the components in the liquid mixture into a gaseous state, the distillation process takes advantage of the different boiling temperatures of the liquid mixture's constituents.
It is significant to highlight that distillation is a physical separation process rather than a chemical reaction.
In several methods for purifying water, distillation is essential. Several desalination facilities use this technique to get drinking water from the ocean.
On an industrial scale, distillation is also used to clean the liquid byproducts of chemical production.
Distillation Machine
Application of Distillation
Recovery of Solvents means distillation makes the process more cost-effective by recovering the expensive solvents frequently employed to separate the active chemical components from crude medicines.
The process of steam distillation is used to extract several volatile oils, including clove oil, fennel oil, and others.
Production of official preparations shows distillation techniques are used to create official preparations, such as distilled water and water for injection.
Distillation techniques can create pure items, such as Absolute alcohol.
Elixirs and other formulations can have their alcohol content measured using distillation techniques for quality control.
Conclusion
Components are separated via the distillation process based on their respective boiling points. The temperatures of substances like regular gas, diesel, and jet fuel differ. Mixed feed streams are separated into different products using distillation columns. The distillation process becomes more difficult the closer the boiling point differences. Distillation takes advantage of the fact that each substance in a solution has a distinct boiling point.
FAQs on Uses of Distillation in Everyday Life and Science
1. What are the most common practical uses of distillation?
Distillation is a fundamental separation technique with many practical applications. Some of the most common uses include:
- Water Purification: It is widely used to desalinate seawater and remove impurities like salts, heavy metals, and microorganisms to produce distilled, potable water.
- Alcoholic Beverages: The process is essential for increasing the alcohol concentration in beverages like whisky, rum, and vodka by separating alcohol from the fermented mixture.
- Petroleum Refining: Crude oil is separated into its various useful components, such as gasoline, diesel, and kerosene, using fractional distillation.
- Essential Oils and Perfumes: Steam distillation is used to extract volatile aromatic compounds from plants and flowers to create essential oils for perfumes and aromatherapy.
2. What is simple distillation and where is it used?
Simple distillation is a technique used to separate a pure liquid from a solution containing a non-volatile solute (like salt in water) or to separate two liquids with a significant difference in their boiling points (typically >25°C). The liquid with the lower boiling point evaporates first, and its vapour is then cooled and condensed back into a pure liquid, leaving the other component behind.
3. What are the main types of distillation used in chemistry and industry?
Besides simple distillation, several specialised types are used for specific purposes:
- Fractional Distillation: Used to separate complex mixtures of liquids with close boiling points, such as refining crude oil.
- Steam Distillation: Used for purifying temperature-sensitive organic compounds, like extracting essential oils, by passing steam through the mixture.
- Vacuum Distillation: Used for separating compounds that have very high boiling points or decompose at high temperatures. Lowering the pressure reduces the boiling point.
4. Why is the difference in boiling points so crucial for distillation to work?
The entire principle of distillation relies on the difference in the volatility, or boiling points, of the components in a mixture. When heated, the substance with the lower boiling point turns into vapour more easily. This vapour is then separated and condensed. If the boiling points are too close, both substances will vaporise at nearly the same temperature, making it impossible to achieve a clean separation through simple distillation.
5. Is distillation considered a physical or a chemical process?
Distillation is a physical process. It involves changes in the physical state of a substance—from liquid to gas (evaporation) and back to liquid (condensation). No new chemical substances are formed during this process. For example, when saltwater is distilled, the H₂O molecules are separated from the salt, but they remain H₂O molecules throughout.
6. How does fractional distillation achieve what simple distillation cannot?
Fractional distillation can separate liquids with very close boiling points because it uses a fractionating column. This column is packed with glass beads or rings, providing a large surface area. As the mixed vapours rise, they cool, condense, and re-vaporise multiple times on these surfaces. With each cycle, the vapour becomes more enriched in the component with the lower boiling point, allowing for a much more precise separation than the single vaporisation-condensation cycle of simple distillation.
7. Can distillation separate a mixture of sugar and water? Why or why not?
Yes, simple distillation can effectively separate sugar and water. This is because sugar is a non-volatile solute, meaning it does not evaporate easily and has a very high decomposition temperature, while water is volatile. When the sugar solution is heated, only the water turns into steam, leaving all the sugar behind. The steam can then be collected and condensed to obtain pure water.









