




Real-World Examples of Hydroiodic Acid in Everyday Life
Kids, do you know that approximately all liquids are either acids or bases? Acids are special types of chemicals. If any chemical has an excess of H+ ions, it is considered an acid. Acids taste sour and are present in many food items. Our favourite lemonade is also an acidic drink. In this article, we will be learning about hydroiodic acid. Hydroiodic acid is a type of acid we all see in industrial uses. It has various uses in industrial manufacturing.
So come, let's learn what hydroiodic acid is and its uses.
Difference Between Acid and Base
What is Hydroiodic Acid?
The aqueous solution of hydrogen iodide (HI) is known as hydroiodic acid. Hydroiodic acid is a strong acid completely ionised or dissociated in any aqueous solution. A colourless gas, hydrogen iodide HI, is dissolved in water to form hydroiodic acid, a pale yellow watery solution. Hydroiodic is a powerful reducing agent and is a highly corrosive acid.
Hydroiodic acid has the property of either losing a proton or taking it back during chemical reactions, and due to this, it is found in many chemical-based applications. The fumes of this acid irritate the eyes and skin and can corrode animal tissues. The gas hydrogen iodide is non-flammable and can be toxic if inhaled. Inhalation may lead to adverse health effects.
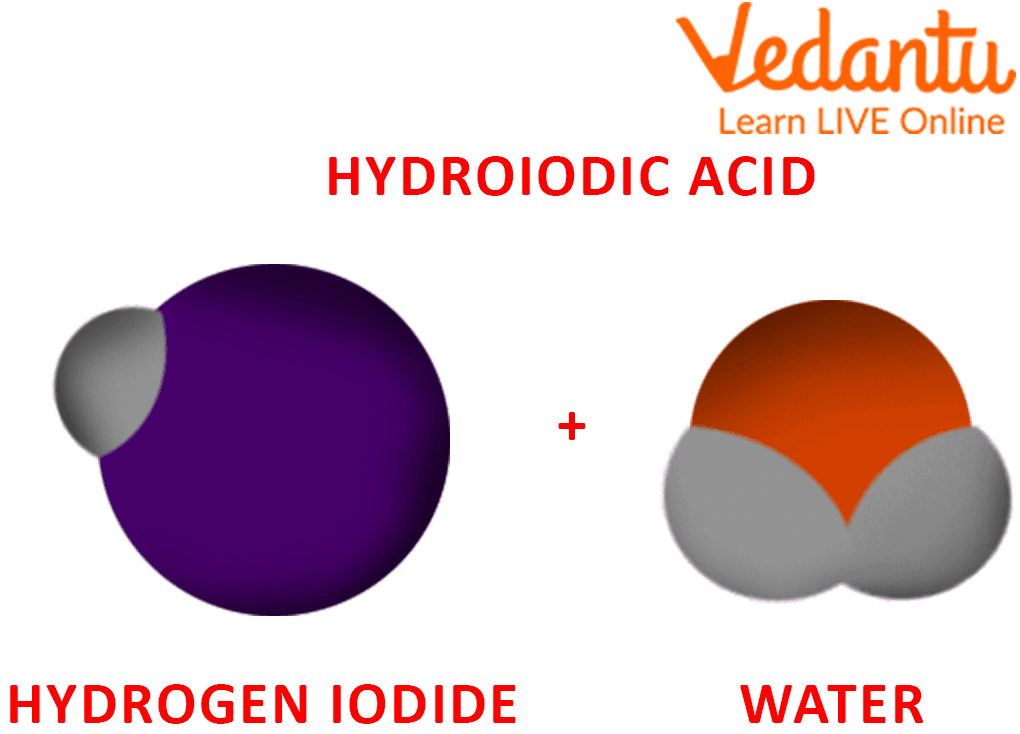
Formation of Hydroiodic Acid
Important Points About Hydroiodic Acid
Kids, let's see some important points about hydroiodic acid:
Chemical Formula: HI
Molecular Mass: 128 gram/mole
Density: 17,00 gram/(centimetre)3
Melting Point: -51°C
Boiling Point: -35.4°C
Appearance: Colours liquid
Solubility: Soluble in water

Hydroiodic Acid in a Breaker
General Uses of Hydroiodic Acid
As we learned about what hydroiodic acid is in the above section, let's learn what are some general uses of hydroiodic acid:
Hydroiodic acid is used as a catalyst in many chemical reactions. A catalyst is any substance which accelerates the rate of chemical reaction without actually being used in the reaction. Hydroiodic acid is used as a catalyst in the formation of acetic acid. Acetic acid is used to produce vinegar that is usually found as an ingredient in our kitchens.
Hydroiodic acid is one of the most expensive catalysing reagents because it is very commonly used in both inorganic as well as organic iodide preparations. This is because of its strong reducing ability.
Hydroiodic acid is also used in the disinfectant industry due to its high level of acidity. The high levels of acidic nature allows hydroiodic acid to kill various types of germs and other microbes on the floor or any other surface.
Hydroiodic acid is also a major component used in medical disinfectants. It is used to sterilise medical tools and also has a chemical composition in liquids used to control microbial growth.
Hydroiodic acid also acts as a pharmaceutical intermediate. The chemicals which are added in any other chemical mixture to make it react and turn into another compound are pharmaceutical intermediates.
For this, it is used in the syrup form as the acid is highly unstable. It is used to manufacture many medicines like malarial infections and chronic bronchitis.
Along with beneficial uses, hydroiodic acid is also used for non-beneficial uses. Due to its unexceptional catalysing property, hydroiodic acid can be used to produce drugs in large amounts.
Hydroiodic acid is also used in the production of an addictive drug. Known as 'meth' or 'ice', methamphetamine is an illegal drug. It is produced by mixing hydroiodic acid with red phosphorus and other compounds.

Hydroiodic Acid as a Disinfectant
Summary
In this article, we learned that hydroiodic acid is produced from a highly toxic gas, hydrogen iodide. It is a pale, colourless liquid and is a powerful reducing agent. Hydroiodic acid is an essential part of chemical-based industries. Due to its unique acidic properties, hydroiodic acid is used to prepare various household and medical disinfectants.
We also learned that hydroiodic acid is useful for humans in many ways, but there also exist some illegal uses for this strong acid. It is also used in the production of various illegal and addictive drugs. Intake of hydroiodic acid can be fatal and can result in immediate death. It can also result in eye damage and severe skin burn. So kids, remember, always be careful with these dangerous acids. Never try to experiment with them without having any adult guidance.
FAQs on Top Uses of Hydroiodic Acid Explained for Students
1. What are some other strong acids rather than hydroiodic acid?
Hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3) and Hydrobromic acid (HBr) are some other strong acids than hydroiodic acid.
2. What is a base?
Base are the chemicals which can neutralise an acid. They are substances which can accept hydrogen ions and can donate hydroxide ions. They are slippery in nature and bitter in taste.
3. Is water an acid?
Water is a compound which is neither an acid nor a base. Water is a substance with a pH of 7, which makes it the neutral substance. Also, reactions of acids and bases produce water as a by-product.
4. What is a pH scale and why is it used?
pH scale is a measure of how acidic or basic a substance is. The pH scale is used to determine the range of acidic or basic composition of a substance. It ranges from 0 to 14 and compounds below 7 are acids and above 7 are basic. The pH range of water is 7, making it a neutral compound.









