




Introduction to Aqueous Tension
Surface tension is the property of a liquid's surface that allows it to resist an external force due to the cohesive nature of its molecular structure. Aqueous tension is defined as "the partial pressure of water vapour in the moist gas." In other words, aqueous tension is equal to the pressure exerted by saturated water vapour. It is the partial pressure of the water vapour present in the moist gas.
Aqueous tension of water depends on the.
In this article, we will come across the definition of aqueous tension, its formula and what are the factors which affect the aqueous tension of water.
What is Aqueous Tension?
The partial pressure of water vapour in a moist gas is defined as aqueous tension. The pressure exerted by moist gas equals the sum of dry and moist vapour partial pressures. Due to the existence of a little amount of water vapour produced by evaporation, the gas turns wet when it is collected over water. Due to the existence of a little amount of water vapour produced by evaporation, the gas turns wet when it is collected over water.
When a gas on the surface of a liquid becomes moist due to the presence of water vapour, this is referred to as evaporation.
Aqueous Tension Formula
Since there is a little amount of water vapour present from evaporation when the gas is gathered over the water, it becomes moist.
The formula for aqueous tension is as follows:
The pressure exerted by moist gas equals the pressure exerted by dry gas plus the pressure exerted by water vapour (aqueous tension).
Mathematically:
$\begin{align} &P_{\text {moist }}=P_{\text {dry }}+f(\text { aqueous tension }) \\ &P_{\text {dry }}=P_{\text {moist }}-\mathrm{f} \end{align}$
In order to obtain the pressure of the dry gas, aqueous tension should be subtracted from the overall pressure.
'Dalton's Law of Partial Pressure' can be used to calculate the pressure exerted by a gas after adjusting for aqueous tension.
$P_{\text {total }}=P_{\text {dry }}+P_{\text {water vapour }}$
In which, Pwatervapour = Aqueous Tension.
Aqueous Tension of Water Depends on Temperature
Aqueous tension is the term used to describe the partial pressure of water vapour in a moist gas. At a specific temperature, a gas will exert aqueous pressure, which is dependent on temperature. Due to the presence of a small amount of water vapour produced by evaporation, when the gas is collected over the water, it becomes moist. Therefore, the pressure of a moist gas equals the sum of the pressure of a dry gas and the pressure of a water vapour (aqueous tension). Therefore, we can say that aqueous tension of water depends on temperature but both pressure and volume have no effect on it.
Partial Pressure of Water Vapour
Gases such as nitrogen and hydrogen are collected over water as shown in the diagram below. The gas is wet and contains water vapour when it is gathered over water. The total pressure exerted by this moist gas is equal to the sum of the partial pressure of the dry gas and the pressure exerted by water vapour. Aqueous tension is another name for the partial pressure of water vapour.
$\begin{align} &\mathrm{P}_{\text {total }}=\mathrm{P}_{\text {gas }}+\mathrm{P}_{\text {water vapour }} \\ &\mathrm{P}_{\text {gas }}=\mathrm{P}_{\text {total }}-\mathrm{P}_{\text {water vapour }} \end{align}$
Actual pressure of gas = Total pressure - Aqueous tension
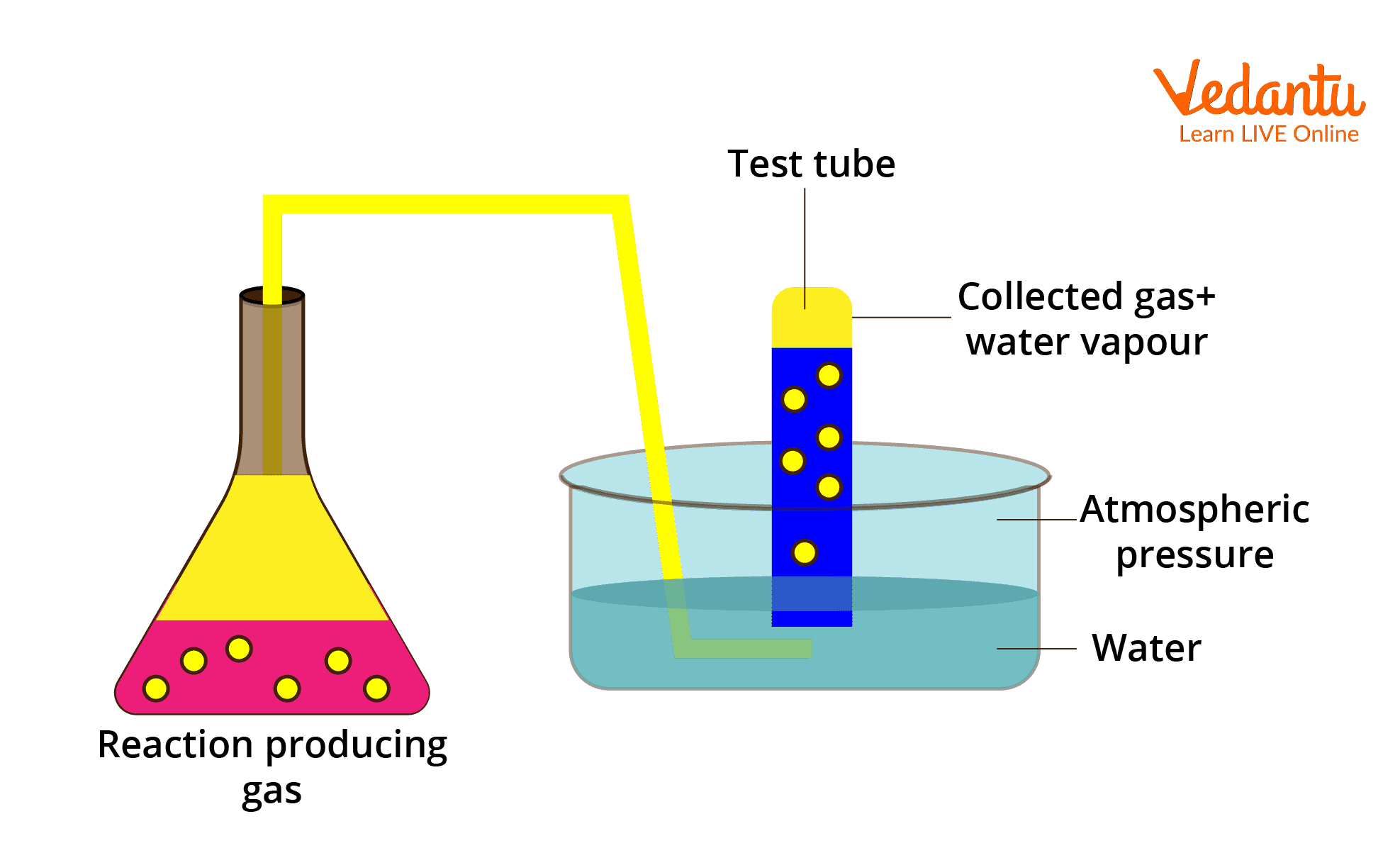
Image : Collection of Gas over Water
Aquavess Water
The aquafina company offers the mineral water bottle known as aquavess water. Produced by Pepsico, it is an American brand. In 1994, Wichita saw the start of its distribution. Reverse osmosis, UV radiation and ozone sterilisation are all used in the purification process for the main unflavored Aquafina product, which is made from local municipal tap water sources.
The chemicals that escape from the bottles into the water are the source of the worries about mineral water in bottles. These consist of plasticizers like Di(2-ethylhexyl)phthalate (DEHP), a well-liked plasticizer that is present in plastic bottles.
Conclusion
Aqueous tension is the partial pressure of the water vapour present in the moist gas.
The pressure of a moist gas is equal to the sum of the pressures of a dry gas and water vapour (aqueous tension).
Mathematically we can say that:
$ P_{\text {total }}=P_{\mathrm{dry}}+\mathrm{P}_{\text {water vapour }}$
We have also seen that aqueous tension is dependent only on the temperature but it is independent of the pressure and volume.
FAQs on Aqueous Tension and Its Formula for JEE
1. What is surface tension?
Surface tension is the tendency for liquid surfaces that are at rest to condense into the smallest surface area. The cgs unit of dyne per centimetre is also used in addition to its SI unit of newton per metre.
Razor blades and insects, which have a density greater than that of water, can float on the surface of the water without even becoming partially immersed because of surface tension.
2. What are the effects of surface tension on water?
The surface tension has the following effects on water:
Raindrops stick to a waxy surface, like a leaf, to form beads. Water congregates into drops because it sticks weakly to wax but firmly to itself. Their nearly spherical shape is due to surface tension.
A tension in the surface between dissimilar liquids is what separates oil and water.
When an object is nonwettable and its weight is low enough to be supported by surface tension forces, it will float if it is denser than water.
3. What are the examples of surface tension ?
The examples of surface tension are:
Small insects, like the water strider, can walk on water because their weight is insufficient to break the surface tension.
The surface tension of water will cause the finely woven material's pores to be bridged, making common tent materials somewhat rainproof.
Surface Tension Disinfectants: Solutions with low surface tension typically serve as disinfectants. This enables them to spread out and disrupt bacterial cell walls.
Droplets and Surface Tension: The shape of liquid droplets is a result of surface tension. The strong interaction of the surface layer tends to pull water droplets into a spherical shape, despite their ease of deformation.

































