




How Does Le Chatelier’s Principle Predict Equilibrium Shifts?
The Le Chatelier's Principle is a fundamental rule in JEE Main Chemistry used to predict how a chemical equilibrium responds to external changes. When a system at equilibrium experiences a shift in concentration, temperature, or pressure, it opposes the change to re-establish balance. This principle simplifies complex equilibrium reactions in predictable, exam-friendly steps and is heavily tested in both concept and application questions for JEE.
Formally, Le Chatelier's Principle states: "If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change." This helps JEE aspirants quickly decide which way a reaction will shift under various disturbances. For instance, increasing the concentration of a reactant will cause the equilibrium to shift towards products, whereas increasing product concentration will shift it towards reactants.
Key Factors Affecting Chemical Equilibrium
- Concentration changes (adding or removing reactants/products)
- Pressure changes (for gaseous systems only)
- Temperature changes (depends on reaction enthalpy)
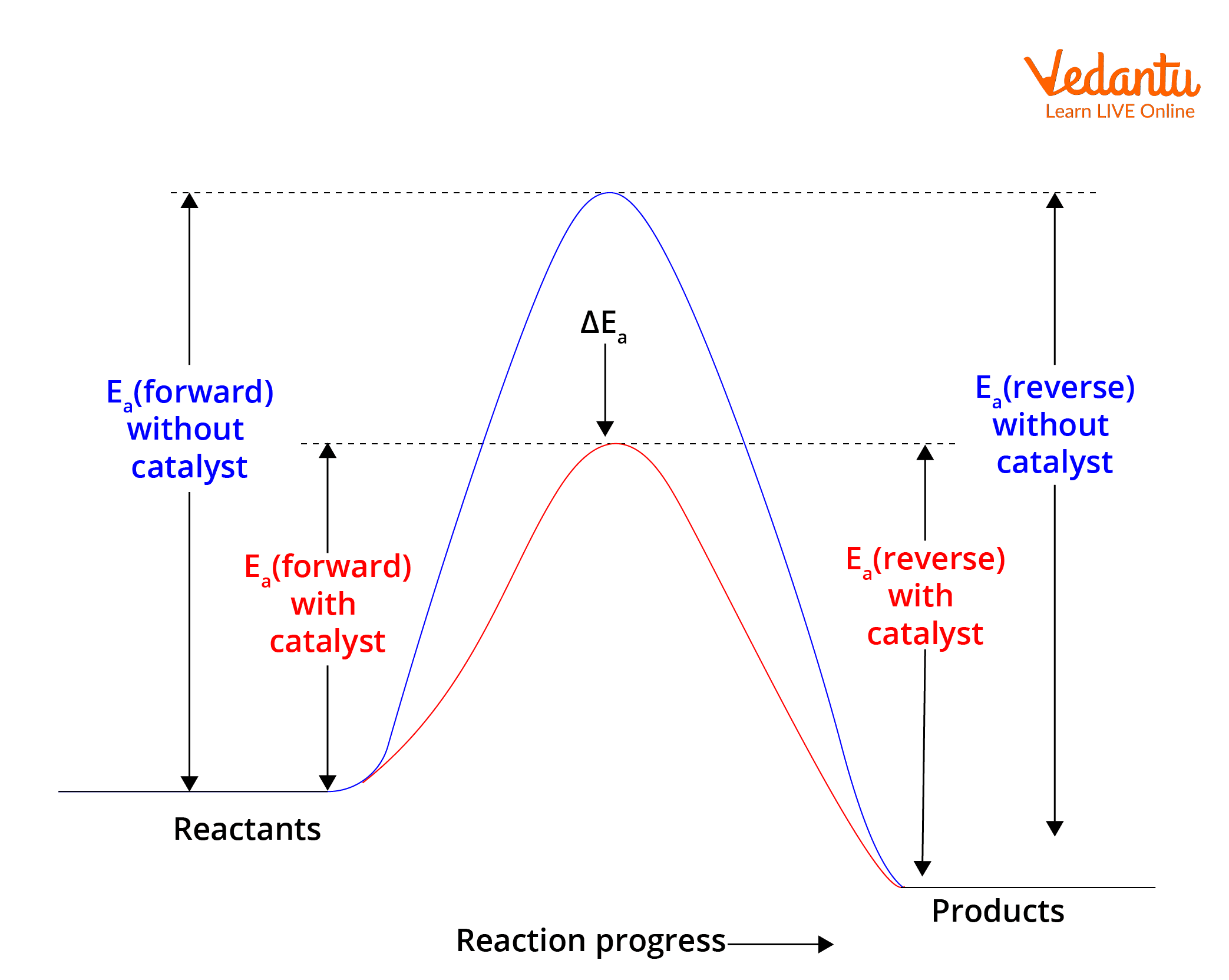
- Catalyst addition (does not affect the position but speeds up equilibrium)
Let’s see how each variable influences the Le Chatelier's Principle with JEE-style examples and highlight common misconceptions.
Effect of Concentration, Temperature, and Pressure: Predicting Equilibrium Shifts
| Change Applied | Equilibrium System Response | Typical Shift Direction | Example |
|---|---|---|---|
| Increase reactant concentration | System consumes added reactant | Shifts right (towards products) | Add H2 in N2 + 3H2 ⇌ 2NH3 |
| Increase product concentration | System consumes added product | Shifts left (towards reactants) | Add NH3 in Haber process |
| Increase pressure (gas reactions) | Favors side with fewer moles of gas | Shifts to fewer moles | N2 + 3H2 ⇌ 2NH3 |
| Increase temperature (exothermic reaction) | Favors endothermic direction | Shifts left | H2 + I2 ⇌ 2HI (ΔH < 0) |
| Catalyst addition | No shift; speeds up both directions | No effect | Used in Haber process |
It is crucial for JEE to realize catalysts do not change the position of equilibrium—only the speed at which equilibration occurs. The chemical equilibrium concept relies on these dynamic balances.
For temperature changes, always identify if the reaction is exothermic (ΔH < 0) or endothermic (ΔH > 0). Increasing temperature favors the endothermic direction; decreasing temperature favors exothermic.
Illustrative Experiments and Real-World Applications
- The Haber process for ammonia: High pressure shifts equilibrium right (N2 + 3H2 ⇌ 2NH3), maximizing yield in industry.
- Chromate-dichromate equilibrium: Adding acids/bases changes the yellow-red color due to shifts in CrO42− ⇌ Cr2O72−.
- CO2 solubility in soda: Opening a bottle lowers pressure, CO2 escapes, shifting equilibrium.
- Contact process for H2SO4 production: Both industrial processes exploit Le Chatelier’s rule for high output.
JEE questions often use these real-life setups to frame application-based MCQs and numericals. Reviewing similar examples in equilibrium mock tests is recommended.
Solving Le Chatelier’s Principle Numericals: Stepwise Method
- Write the balanced equation and identify reactants/products, noting the number of moles (for gases).
- Determine if the reaction is exothermic or endothermic (ΔH known or given).
- Apply the specific change (e.g., pressure increase), and analyze which side the system will favor using the principle.
- Justify the shift: more product, more reactant, fewer/more moles of gas, favoring heat absorption or release.
- Mark the correct direction (left/right/no change) and reason in concise steps.
Example:
For N2(g) + 3H2(g) ⇌ 2NH3(g), increasing pressure favors the right (fewer moles gas); increasing temperature (exothermic) shifts left.
Practice Worksheet: Le Chatelier’s Principle MCQs
- Increasing pressure in 2SO2 + O2 ⇌ 2SO3 shifts equilibrium which way?
- For endothermic CH4 + H2O ⇌ CO + 3H2, what is the effect of raising temperature?
- Adding NaOH to CrO42−/Cr2O72− equilibrium: color change and direction?
- Does catalyst addition affect equilibrium in N2 + 3H2 ⇌ 2NH3?
- If product is removed as formed, how does equilibrium respond?
Download more practice papers and concept revision material on JEE equilibrium practice paper for integrated numericals and MCQs.
Quick Revision: Key Points and Common Traps
- Always identify if changes affect concentration, pressure (only gases), or temperature (based on ΔH).
- Catalyst does not shift equilibrium; it only speeds up attainment.
- Pressure change has no effect if total gas moles are equal on both sides.
- Removing products continuously can drive a reaction nearly to completion.
- Never apply Le Chatelier’s Principle to non-equilibrium or irreversible reactions.
- Mixing up endothermic/exothermic directions is a frequent JEE mistake.
Effect of Catalyst is essential in Le Chatelier’s Principle for JEE. Catalysts do not change equilibrium position but influence reaction speed significantly.
For comprehensive coverage, review dynamic equilibrium, chemical thermodynamics, and chemical kinetics for related concepts that overlap with Le Chatelier's Principle in JEE Main exams.
FAQs on Le Chatelier’s Principle Explained for Chemistry Students
1. What is Le Chatelier's Principle in chemistry?
Le Chatelier's Principle states that if a system at chemical equilibrium is disturbed by a change in conditions—such as concentration, temperature, or pressure—the system shifts in a direction that opposes the change and restores a new equilibrium state.
Key points include:
- If concentration of reactants increases, equilibrium shifts towards products.
- For temperature increase in endothermic reactions, equilibrium shifts towards products.
- Increasing pressure favours the side with fewer gas molecules.
2. How do you predict equilibrium shifts using Le Chatelier’s Principle?
To predict equilibrium shifts, identify which factor is being changed (concentration, pressure, or temperature) and then apply Le Chatelier's Principle to see which direction minimizes that disturbance.
Steps include:
- Check which change occurs (add/removal, increase/decrease).
- Determine if the reaction will favour products or reactants to oppose the change.
- Apply specific rules:
- Increase in reactant → shift to product side.
- Decrease in reactant → shift to reactant side.
- Increase in temperature (endothermic) → shift to product; (exothermic) → shift to reactant.
- Increase in pressure → shift to side with fewer gas molecules.
3. Which factors affect chemical equilibrium according to Le Chatelier?
Chemical equilibrium is influenced by several factors as per Le Chatelier's Principle:
- Concentration of reactants or products
- Temperature of the system
- Pressure/volume (especially for gases)
- Note: Catalysts speed up equilibrium but do not affect its position.
4. How does a change in pressure influence equilibrium position?
A change in pressure mainly affects reactions involving gases. Le Chatelier’s Principle states that if pressure increases, equilibrium shifts toward the side with fewer gaseous molecules; if pressure decreases, it shifts to the side with more gas molecules.
Example: For N₂(g) + 3H₂(g) ⇌ 2NH₃(g), increasing pressure shifts equilibrium to the right (toward NH₃), because 4 gas molecules become 2.
5. Give one real-life example of Le Chatelier’s Principle application.
One classic real-life application is the Haber process for ammonia manufacture:
- By increasing pressure and removing ammonia, manufacturers shift the equilibrium toward more product formation.
- This optimizes yield based on Le Chatelier's Principle.
- It’s also used in laboratory experiments, like chromate-dichromate equilibrium colour changes.
6. What is the effect of temperature on endothermic vs. exothermic reactions in equilibrium?
Temperature changes affect equilibrium differently for endothermic and exothermic reactions:
- Endothermic reaction (ΔH > 0): Increasing temperature shifts equilibrium toward products.
- Exothermic reaction (ΔH < 0): Increasing temperature shifts equilibrium toward reactants.
7. Can catalysts alter the equilibrium position as per Le Chatelier?
Catalysts do not change the equilibrium position; they only help reach equilibrium faster.
- Catalysts lower activation energy for both forward and reverse reactions equally.
- They speed up the attainment of equilibrium but do not favour any particular side.
8. Does Le Chatelier’s Principle apply to reactions in open containers?
Le Chatelier’s Principle strictly applies to closed systems at equilibrium.
- In open containers, products or reactants can escape, preventing true equilibrium from being established.
- Therefore, Le Chatelier's Principle doesn't predict shifts accurately for open systems.
9. What role does inert gas addition play in equilibrium shifts?
Adding an inert gas at constant volume does not affect the position of equilibrium since partial pressures of reactants and products remain unchanged.
- Inert gas increases total pressure but not concentrations of reacting species.
- If added at constant pressure (with volume allowed to change), equilibrium may shift depending on the number of gas molecules.
10. How to avoid common mistakes when predicting equilibrium shifts in MCQs?
To avoid errors, always:
- Read the reaction and given change (concentration, temperature, pressure) carefully.
- Identify whether the reaction is endothermic or exothermic.
- Count total gas molecules on each side for pressure changes.
- Remember catalysts do not shift equilibrium.
- Visualize the ‘opposing change’ logic from Le Chatelier’s Principle.
































