




How Did Rutherford’s Experiment Change Our Understanding of the Atom?
Rutherford Scattering Experiment: Foundations of Atomic Structure
The Rutherford scattering experiment is a milestone in physics that revolutionized our understanding of atomic structure. It provided undeniable evidence that atoms contain a small, dense nucleus, forever changing the way scientists view the microscopic world.
In this experiment, alpha particles—helium nuclei—were directed at a very thin sheet of gold foil. Their paths and deflections revealed secrets about the atom’s internal arrangement, disproving previous models and influencing atomic theory for generations.
Experimental Setup and Key Apparatus
The core setup of Rutherford’s experiment included a radioactive source emitting alpha particles, a thin gold foil target, and a circular zinc sulfide screen that detected scattered particles by emitting tiny flashes of light.
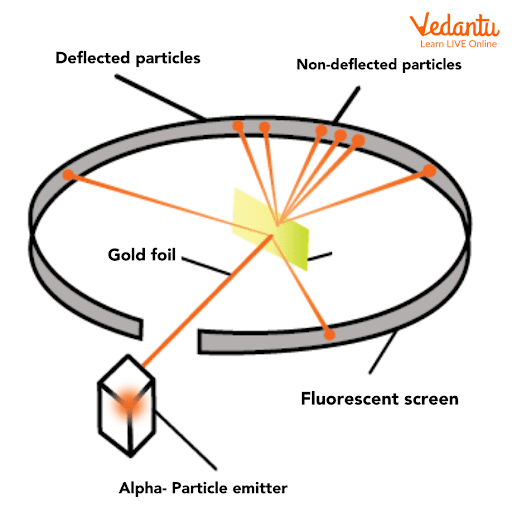
The zinc sulfide screen surrounded the gold foil, allowing the detection of alpha particles at multiple angles. Observers recorded the positions and frequency of the flashes to analyze deflection patterns.
Alpha particles were chosen for the experiment because they are heavy, positively charged, and energetic. This combination made any deflections due to atomic forces more visible and measurable.
Observations: Scattering Patterns and Their Significance
Three major findings highlighted the atomic mystery: Most alpha particles passed straight through the gold foil, a small fraction were deflected at large angles, and an even tinier number bounced almost directly back.
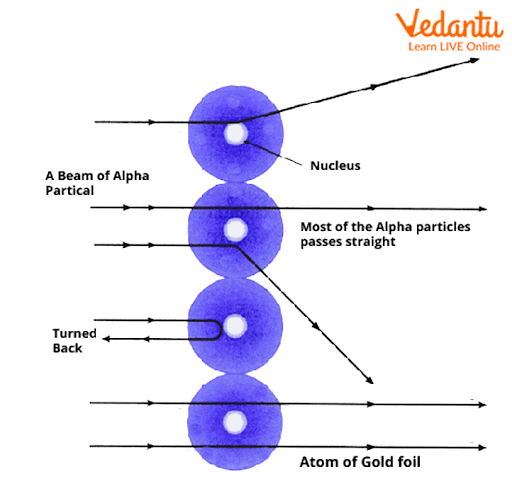
The observation that the vast majority of alpha particles experienced minimal or no deflection indicated that most of the atom is empty space, not filled with positive charge as previously thought.
The rare, sharp deflections of some particles suggested the existence of a very dense and positively charged “core,” now called the nucleus. This core repelled the alpha particles quite forcefully in certain collisions.
Disproving the Plum Pudding Model
Prior to Rutherford’s experiment, the plum pudding model had dominated. It envisioned electrons in a sea of positive charge. Rutherford’s results showed that positive charge is instead sharply localized.
This overturned the old model and paved the way for modern atomic theory, ultimately influencing quantum mechanics, atomic physics, and chemistry. Later improvements led to the quantized model of Bohr and beyond.
Principles and the Rutherford Scattering Equation
The principle behind the scattering experiment rests on the Coulomb force between the positively charged alpha particles and the nucleus. The deflection angle provided information about the electrostatic forces and structure of the atom.
The likelihood of an alpha particle scattering by an angle $\theta$ is described by the Rutherford differential scattering cross-section equation:
$ \dfrac{d\sigma}{d\Omega} = \left( \dfrac{1}{4\pi\epsilon_0} \right)^2 \left( \dfrac{Z_1Z_2e^2}{4E} \right)^2 \csc^4 \left( \dfrac{\theta}{2} \right) $
In this equation, $Z_1$ and $Z_2$ are the atomic numbers, $e$ is the elementary charge, $E$ is the energy of the alpha particle, $\theta$ is the scattering angle, and $\epsilon_0$ is the vacuum permittivity.
Key Concepts Illustrated by Real-Life Analogy
A helpful analogy for the scattering experiment is shooting bullets at a tissue paper containing a few steel balls hidden inside. Most bullets pass through, but a few hit the steel balls and bounce off sharply.
In the atomic context, most alpha particles zip through empty atomic space, but occasionally, one approaches a nucleus closely and is powerfully deflected—just as a bullet would bounce off a steel core.
Comparing Atomic Models: Before and After Rutherford
| Model | Description |
|---|---|
| Plum Pudding | Positive charge spread, electrons within |
| Rutherford | Tiny, dense nucleus with electrons outside |
The shift from the plum pudding model to Rutherford’s nuclear model marked a revolution in our understanding of atomic structure and led to numerous advances in nuclear and quantum physics.
Main Conclusions Drawn from the Experiment
The experiment firmly established that the atom consists of a tiny, concentrated nucleus containing most atomic mass and positive charge, with negatively charged electrons orbiting at great distances.
This model explained not only scattering results, but also atomic properties such as stability and the arrangement of electrons. Its impact was profound across all of modern physics.
Applications and Limitations of Rutherford Scattering
- The method is used in nuclear physics to probe nuclear size
- Introduced the concept of the nucleus for chemical reactions
- Inspiration for advancements in quantum mechanics and spectroscopy
Limitations exist: the model failed to explain atomic stability and electron energy levels. Subsequent models, like Bohr’s, addressed these gaps using quantization postulates.
Numerical Example: Calculating a Scattering Angle
Suppose an alpha particle with energy $8 \, \text{MeV}$ approaches a gold nucleus $(Z=79)$ and is found to be scattered by $60^\circ$. Find the differential cross-section at this angle.
Known values: $Z_1 = 2$ (alpha), $Z_2 = 79$ (gold), $e = 1.6 \times 10^{-19} \, \text{C}$, $\epsilon_0 = 8.85 \times 10^{-12} \, \text{C}^2/(\text{N}\cdot\text{m}^2)$, $E = 8 \times 10^6 \, \text{eV}$, $\theta = 60^\circ$.
The formula is:
$ \dfrac{d\sigma}{d\Omega} = \left( \dfrac{1}{4\pi\epsilon_0} \right)^2 \left( \dfrac{Z_1Z_2e^2}{4E} \right)^2 \csc^4 \left( \dfrac{\theta}{2} \right) $
Substitute the values:
$ \dfrac{d\sigma}{d\Omega} = \left( \dfrac{1}{4\pi(8.85 \times 10^{-12})} \right)^2 \left( \dfrac{2 \times 79 \times (1.6 \times 10^{-19})^2}{4 \times 8 \times 10^6 \times 1.6 \times 10^{-19}} \right)^2 \csc^4 30^\circ $
Simplify the csc term:
$\csc 30^\circ = 2$, so $\csc^4 30^\circ = 16$.
Final answer:
The value of $\dfrac{d\sigma}{d\Omega}$ will be a very small number, indicating the probability of scattering at $60^\circ$ is much smaller than at smaller angles. This matches Rutherford’s findings.
Practice Question for Exam Preparation
If the gold foil in Rutherford’s experiment were replaced with aluminum, how would the scattering pattern change? Briefly justify your answer based on nuclear charge and atomic structure.
Common Mistakes When Studying Rutherford Scattering
A common mistake is misunderstanding why most alpha particles pass through. It is due to the enormous empty space within atoms and the tiny, concentrated nucleus—not because the nucleus is very weak or diffuse.
Another pitfall involves misapplying the Rutherford equation at high energies or for light elements, where quantum effects make classical scattering models invalid. Such situations require quantum corrections for accuracy.
Related Physics Topics
- Explore current loops and fields at Magnetic Effects Of Current And Magnetism
- Deeper look at the atom in Atomic Structure
- See nuclear energy insights at Nuclear Reactor
- Understand particle behavior with Kinetic Theory Of Gases
- Study heat, work, and disorder in Thermodynamics
- Review the basics of forces in Laws Of Motion
FAQs on What Is the Rutherford Scattering Experiment?
1. What is the Rutherford scattering experiment?
The Rutherford scattering experiment was a landmark experiment conducted by Ernest Rutherford in 1909 to investigate the structure of the atom. It involved passing alpha particles through a thin gold foil and observing their scattering patterns. The key observations led to the discovery that most of the atom is empty space and the existence of a small, dense, positively charged nucleus.
2. What were the main observations of the Rutherford gold foil experiment?
The main observations of the Rutherford gold foil experiment are as follows:
- Most alpha particles passed straight through the gold foil with little or no deflection.
- A small fraction of alpha particles were deflected by large angles.
- Very few alpha particles (about 1 in 8000) bounced back nearly towards the source.
3. What conclusions did Rutherford draw from his scattering experiment?
From the scattering experiment, Rutherford concluded that:
- The atom is mostly empty space.
- The atom contains a small, dense, and positively charged nucleus at its center.
- The electrons revolve around the nucleus.
4. Why was gold foil used in Rutherford's experiment?
Gold foil was used in Rutherford's experiment because:
- Gold can be hammered into extremely thin sheets, only a few atoms thick, allowing alpha particles to pass through with minimal obstruction.
- It minimizes the chances of multiple collisions, making it easier to observe single scattering events.
5. How did Rutherford’s experiment disprove the plum pudding model?
Rutherford's experiment disproved the plum pudding model by showing that:
- Alpha particles were sometimes deflected at large angles or bounced back, which was impossible if positive charge were spread uniformly as J.J. Thomson's model proposed.
- Instead, the results indicated a small, dense, and centralized nucleus, contradicting the idea of a diffused positive charge cloud.
6. What is the significance of the atomic nucleus according to Rutherford?
According to Rutherford, the atomic nucleus is significant because:
- It contains nearly all the mass of the atom.
- It is positively charged and occupies a very small volume at the center of the atom.
- It determines the atom's identity and most of its physical properties.
7. What are the limitations of Rutherford’s atomic model?
The limitations of Rutherford's atomic model include:
- It could not explain the stability of atoms; according to classical physics, revolving electrons should lose energy and spiral into the nucleus.
- It failed to account for the observed atomic spectra, especially the line spectra emitted by atoms.
- It did not describe electron arrangement or energy levels.
8. What are alpha particles, and why were they used in Rutherford’s experiment?
Alpha particles are positively charged particles consisting of two protons and two neutrons. They were used in Rutherford's experiment because:
- They have enough mass and positive charge to interact strongly with the atomic nucleus.
- Their straight paths make deflections noticeable, helping to infer the atomic structure from scattering patterns.
9. Explain the scattering pattern of alpha particles observed in Rutherford’s experiment.
The scattering pattern of alpha particles showed:
- Most passed straight through the gold foil — indicating empty space in the atom.
- Some were deflected at small angles — suggesting repulsion by a positively charged center.
- A very small number were deflected back — proving the presence of a dense nucleus.
10. Describe the main features of Rutherford's nuclear model of the atom.
The main features of Rutherford's nuclear model are:
- There is a tiny, dense, positively charged nucleus at the atom's center.
- Electrons revolve around this nucleus.
- Most of the atom's volume is empty space.
- The size of the nucleus is much smaller than the atom as a whole.
























