
2-Chloro-2-methylbutane on reaction with sodium methoxide in methanol yields
a.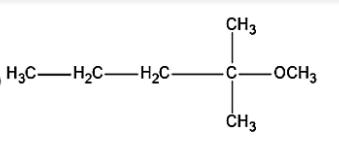
b. 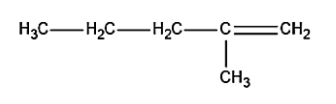
c. 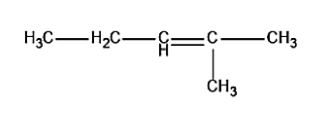
(A) All of these
(B) (a) and (c)
(C) (c)only
(D) (a) and (b)
Answer
232.8k+ views
Hint: When 2-Chloro-2-methylbutane reacts with sodium methoxide in methanol, it undergoes \[{S_N}1\] and \[{E_1}\]. \[{S_N}1\]means unimolecular nucleophilic substitution reaction and \[{E_1}\]means unimolecular elimination reaction. On knowing what type of reaction can take place in this \[{S_N}1\]and \[{E_1}\]mechanism we can determine the product.
Complete step by step answer:
2-Chloro-2-methylbutane when reacted with sodium methoxide in methanol, it will form a carbocation by the removal of \[NaCl\]. The formed carbocation will undergo \[{S_N}1\] and \[{E_1}\].
In \[{S_N}1\]mechanism, nucleophile will come and attack the same position of the leaving group, that means that the nucleophile, methoxy group \[C{H_3}{O^ - }\] will be attached in the position of the leaving chlorine \[C{l^ - }\] group. Thus, leading to the formation of 2-Methoxy-2-methylbutane i.e. compound (a) in the figure.
In the\[{E_1}\] mechanism, the deprotonation will occur in the carbon atom which is nearer to the carbocation and hence leading to the formation of alkene. In 2-methylbutane carbocation, there are two carbon atoms which are nearer to the carbocation. One carbon atom is at the terminal position and the other carbon atom is at the third position.
In the terminal carbon atom, a proton is removed, thus leading to the formation of 2-methyl-1-butene i.e. compound (b) in the figure.
In the third carbon atom, a proton will be removed, thus leading to the formation of 2-methyl-2-butene i.e. compound (c) in the figure.
Therefore, all the three compounds(a), (b) and (c) during the reaction of 2-Chloro-2-methylbutane with sodium methoxide in methanol.
Hence, the correct answer is option (A) all the above.
Additional information:
Comparison of \[{S_N}1\] and \[{S_N}2\]
Note: In the reaction both elimination and substitution reaction take place. In the elimination reaction the deprotonation of the carbocation takes places to form alkene, while in the substitution reaction, substitution of leaving group by a nucleophile takes place. Here substitution takes place at only one position while elimination is taking place at two positions.
Complete step by step answer:
2-Chloro-2-methylbutane when reacted with sodium methoxide in methanol, it will form a carbocation by the removal of \[NaCl\]. The formed carbocation will undergo \[{S_N}1\] and \[{E_1}\].
In \[{S_N}1\]mechanism, nucleophile will come and attack the same position of the leaving group, that means that the nucleophile, methoxy group \[C{H_3}{O^ - }\] will be attached in the position of the leaving chlorine \[C{l^ - }\] group. Thus, leading to the formation of 2-Methoxy-2-methylbutane i.e. compound (a) in the figure.
In the\[{E_1}\] mechanism, the deprotonation will occur in the carbon atom which is nearer to the carbocation and hence leading to the formation of alkene. In 2-methylbutane carbocation, there are two carbon atoms which are nearer to the carbocation. One carbon atom is at the terminal position and the other carbon atom is at the third position.
In the terminal carbon atom, a proton is removed, thus leading to the formation of 2-methyl-1-butene i.e. compound (b) in the figure.
In the third carbon atom, a proton will be removed, thus leading to the formation of 2-methyl-2-butene i.e. compound (c) in the figure.
Therefore, all the three compounds(a), (b) and (c) during the reaction of 2-Chloro-2-methylbutane with sodium methoxide in methanol.
Hence, the correct answer is option (A) all the above.
Additional information:
Comparison of \[{S_N}1\] and \[{S_N}2\]
| \[{S_N}1\] | \[{S_N}2\] |
| Unimolecular | Bimolecular |
| Rate depends on two species | Rate depends only on one species |
| Second order | First order |
| Solvent used is polar protic | Solvent used is Polar aprotic |
| Alkyl halide reacts in the order\[3^\circ > 2^\circ > 1^\circ \] | Alkyl halide reacts in the order\[1^\circ > 2^\circ > 3^\circ \] |
Note: In the reaction both elimination and substitution reaction take place. In the elimination reaction the deprotonation of the carbocation takes places to form alkene, while in the substitution reaction, substitution of leaving group by a nucleophile takes place. Here substitution takes place at only one position while elimination is taking place at two positions.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




