
Amylopectin is a polymer of:
(A) $\beta $ D glucose
(B) $\alpha $ D glucose
(C) $\beta $ D fructose
(D) $\alpha $ D fructose
Answer
233.1k+ views
Hint:
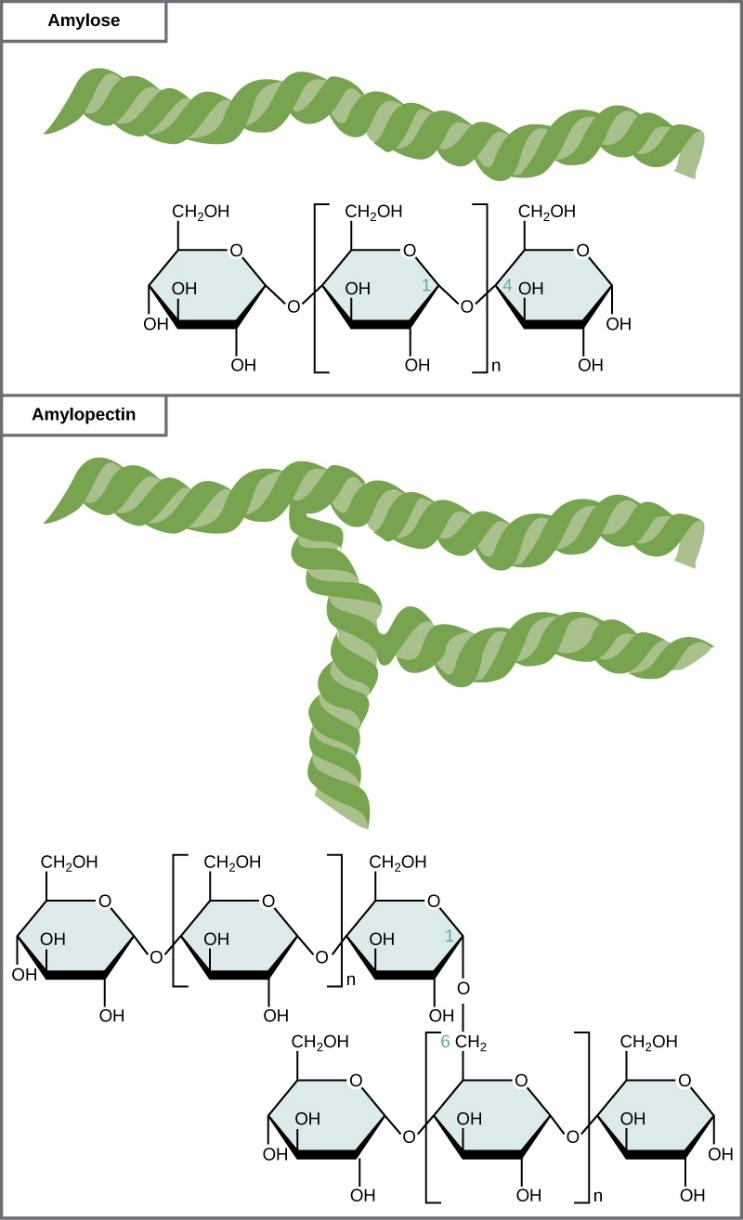
Amylopectin is a water soluble polysaccharide and highly branched polymer. It is one of the two components of starch, the other being amylose. Glucose units are linked in a linear way with $\alpha $ glycosidic bonds.
Complete step by step answer:
Starch is a colorless and odorless polysaccharide that is found in plants as stored carbohydrates. It is a polymer of glucose monomers that are linked with each other to form polysaccharide. It is composed of two types of polysaccharide molecules:
A. Amylose
B. Amylopectin
Now, we will discuss amylopectin.
Amylopectin is a polymer of several D-glucose molecules. $80\% $ of amylopectin is present in starch.
Further, the amylopectin molecules are linked by $\alpha 1,4$-glycosidic bonds and $\alpha 1,6 - $ glycosidic bonds. When iodine is added to starch, it gives reddish-brown appearance due to the presence of amylopectin. It readily dissolves in hot water.
Hence, option B is correct.
Note: Polysaccharides are large molecules that feature high molecular weights and contain hundreds of glucose units joined by $\beta $ glycosidic bonds between C-$1$ and C-$4$ sites of adjacent sugars. The most important polysaccharides are cellulose, starch and glycogen.
Amylopectin is a water soluble polysaccharide and highly branched polymer. It is one of the two components of starch, the other being amylose. Glucose units are linked in a linear way with $\alpha $ glycosidic bonds.
Complete step by step answer:
Starch is a colorless and odorless polysaccharide that is found in plants as stored carbohydrates. It is a polymer of glucose monomers that are linked with each other to form polysaccharide. It is composed of two types of polysaccharide molecules:
A. Amylose
B. Amylopectin
Now, we will discuss amylopectin.
Amylopectin is a polymer of several D-glucose molecules. $80\% $ of amylopectin is present in starch.
Further, the amylopectin molecules are linked by $\alpha 1,4$-glycosidic bonds and $\alpha 1,6 - $ glycosidic bonds. When iodine is added to starch, it gives reddish-brown appearance due to the presence of amylopectin. It readily dissolves in hot water.

Hence, option B is correct.
Note: Polysaccharides are large molecules that feature high molecular weights and contain hundreds of glucose units joined by $\beta $ glycosidic bonds between C-$1$ and C-$4$ sites of adjacent sugars. The most important polysaccharides are cellulose, starch and glycogen.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




