
Arrange in basic order of their basic strength
I. 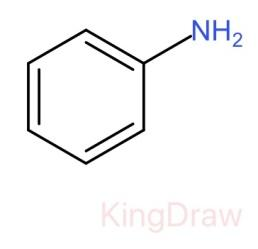
II. 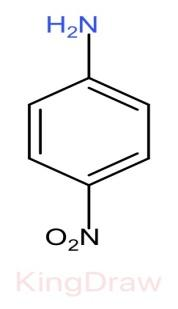
III. 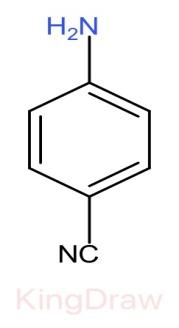
IV. 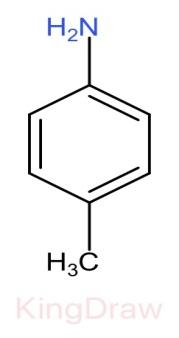
(a) I>II>III>IV
(b) II>III>I>IV
(c) IV>I>III>II
(d) IV>I>II>III
Answer
233.1k+ views
Hint: Basicity can be referred to as "electron pair instability", and this electron pair instability increases as the charge density on the aniline increases. This means that the basicity should or does increase with the increased negative charge on the group.
Complete answer:
Now we know that basicity increases with the charge density on the group, therefore, we can say that a conjugate base of an amine always be stronger than the amine itself. This relationship between the lower basicity due to the lower charge densities also applies to the lone pairs that are being localised into a larger pi system through resonance.
Thus, we can say that the stronger the acid, the weaker the conjugate base or vice versa.
* In case of the given question, if an electron withdrawing group is attached to amine like ${C}_{6}{H}_{5}$- groups, they decrease the electron density on the nitrogen atom hence decreasing the basicity.
* Further, the effect of an electron withdrawing group like, $N{O}_{2}$, $CN$, $C{H}_{3}$, near to a basic center decreases the basicity due to the electron withdrawing inductive effect.
* Now, among the groups $N{O}_{2}$, $CN$, $C{H}_{3}$, $C{H}_{3}$ has the least electron withdrawing capacity and hence has more basic strength than $N{O}_{2}$, $CN$.
* Among $N{O}_{2}$, $CN$, $N{O}_{2}$ has more electron withdrawing capacity than $CN$, but it also shows resonance in its structure which provides stabilization hereby decreasing the basicity. Thus, $CN$ has more basic strength than $N{O}_{2}$.
Therefore, the correct order of the basic strength is IV>I>III>II. Hence (c) is the correct answer
Note: When a nitro group is present at the o or p-positions then the electron withdrawal is increased for the interaction of the unshared pair electron of nitrogen in the amino group with the benzene nucleus having delocalised p orbital.
Complete answer:
Now we know that basicity increases with the charge density on the group, therefore, we can say that a conjugate base of an amine always be stronger than the amine itself. This relationship between the lower basicity due to the lower charge densities also applies to the lone pairs that are being localised into a larger pi system through resonance.
Thus, we can say that the stronger the acid, the weaker the conjugate base or vice versa.
* In case of the given question, if an electron withdrawing group is attached to amine like ${C}_{6}{H}_{5}$- groups, they decrease the electron density on the nitrogen atom hence decreasing the basicity.
* Further, the effect of an electron withdrawing group like, $N{O}_{2}$, $CN$, $C{H}_{3}$, near to a basic center decreases the basicity due to the electron withdrawing inductive effect.
* Now, among the groups $N{O}_{2}$, $CN$, $C{H}_{3}$, $C{H}_{3}$ has the least electron withdrawing capacity and hence has more basic strength than $N{O}_{2}$, $CN$.
* Among $N{O}_{2}$, $CN$, $N{O}_{2}$ has more electron withdrawing capacity than $CN$, but it also shows resonance in its structure which provides stabilization hereby decreasing the basicity. Thus, $CN$ has more basic strength than $N{O}_{2}$.
Therefore, the correct order of the basic strength is IV>I>III>II. Hence (c) is the correct answer
Note: When a nitro group is present at the o or p-positions then the electron withdrawal is increased for the interaction of the unshared pair electron of nitrogen in the amino group with the benzene nucleus having delocalised p orbital.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




