
How many carbon-hydrogen bond orbitals are available for overlap with the vacant p-orbital in ethyl carbocation?
(a) 0
(b) 3
(c) 5
(d) 6
Answer
232.8k+ views
Hint: Carbocation is a species in which the carbon atom has a positive charge and can form three bonds. Stability of carbocation depends on the number of carbon atoms and functional groups attached to it.
Complete step by step solution:
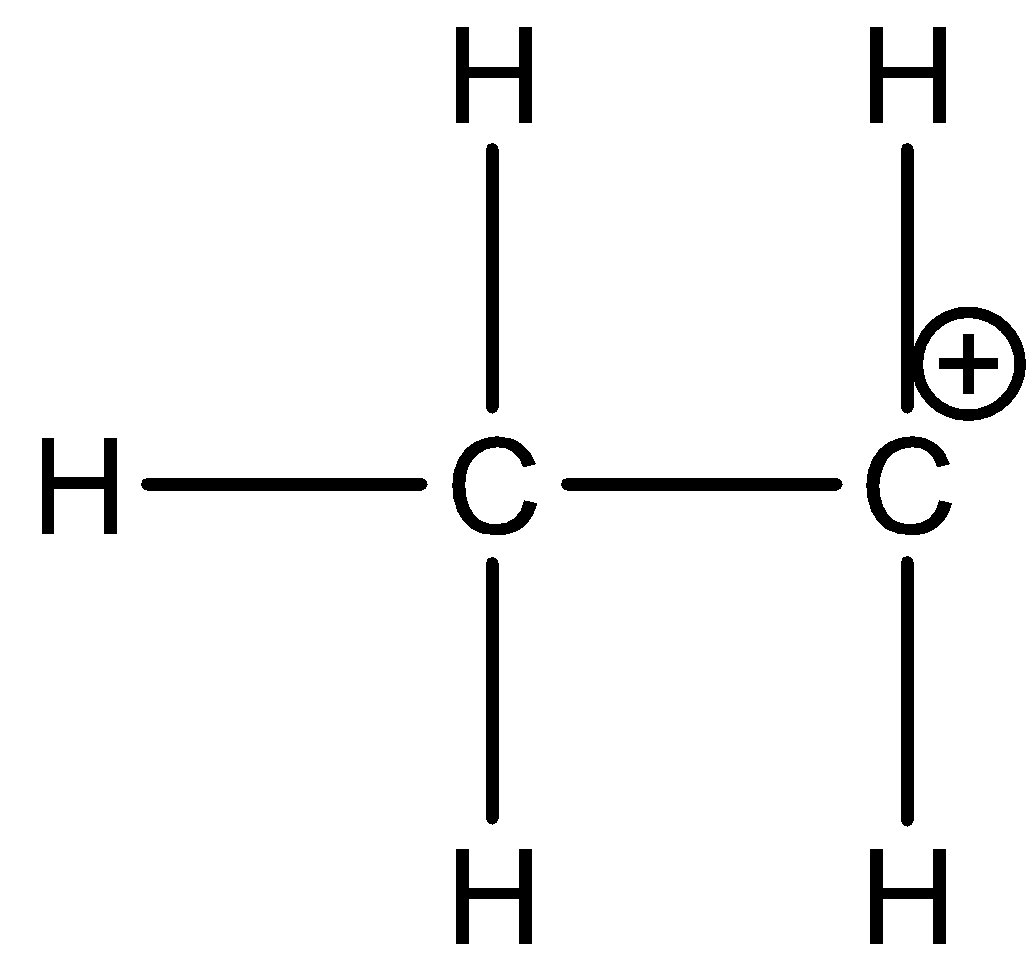
-Ethyl carbocation is ethane in which one of the hydrogen is missing from one carbon and it has a positive charge on that carbon atom. The structure of ethyl carbocation is as shown in the figure below
-Now let us see how many p orbitals are there vacant in the carbocation.
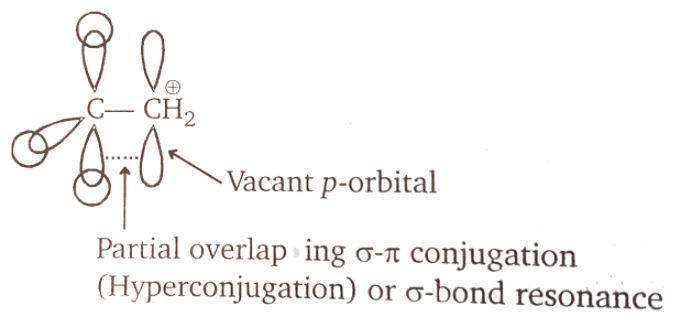
There are two vacant orbitals. The carbon next to carbocation has carbon-hydrogen bonds. All the three are available for the overlap with the vacant orbital of the carbocation. The vacant p-orbital forms a partial overlap giving a partial sigma-pi conjugation. This can also lead to hyperconjugation. Hyper conjugation is the interaction of the electrons in a sigma bond (C-H or C-C) with adjacent empty or partially filled p orbital or pi orbital and it gives an extended molecular orbital which increases the stability of the system. It is not resonance.
Hence, the 3 carbon-hydrogen bonds are available for overlap with the vacant p-orbital of the ethyl carbocation. The correct answer to the question is option (b).
Note: Due to the vacant p orbital, carbocation is electron deficient. Because of this, it can act as an electrophile. Carbocation has a trigonal planar shape.
Complete step by step solution:
-Ethyl carbocation is ethane in which one of the hydrogen is missing from one carbon and it has a positive charge on that carbon atom. The structure of ethyl carbocation is as shown in the figure below

-Now let us see how many p orbitals are there vacant in the carbocation.

There are two vacant orbitals. The carbon next to carbocation has carbon-hydrogen bonds. All the three are available for the overlap with the vacant orbital of the carbocation. The vacant p-orbital forms a partial overlap giving a partial sigma-pi conjugation. This can also lead to hyperconjugation. Hyper conjugation is the interaction of the electrons in a sigma bond (C-H or C-C) with adjacent empty or partially filled p orbital or pi orbital and it gives an extended molecular orbital which increases the stability of the system. It is not resonance.
Hence, the 3 carbon-hydrogen bonds are available for overlap with the vacant p-orbital of the ethyl carbocation. The correct answer to the question is option (b).
Note: Due to the vacant p orbital, carbocation is electron deficient. Because of this, it can act as an electrophile. Carbocation has a trigonal planar shape.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




