




Introduction to Elimination Reactions
Elimination reactions are those reactions in which two substituents are eliminated from the molecule, and a new C – C double bond is formed between the two carbon atoms to which the eliminated atoms or groups were previously attached. Elimination reaction is always accompanied by a change in hybridisation of the molecule involved in the reaction. These are the reverse of the addition reaction. In an elimination reaction, two atoms or groups (YZ) are removed from the substrate with the formation of pi bonds.
Types of Elimination Reaction
Depending on the reagents and conditions involved, an elimination reaction may be first order (E1) or second order (E2).
Example of E1 Reaction:
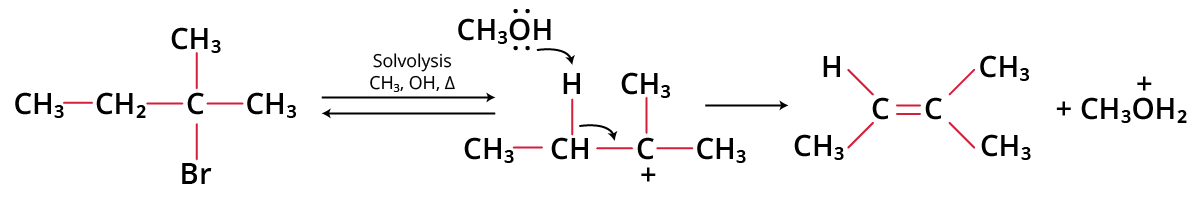
Example of E1 Reaction
Example of E2 Reaction:
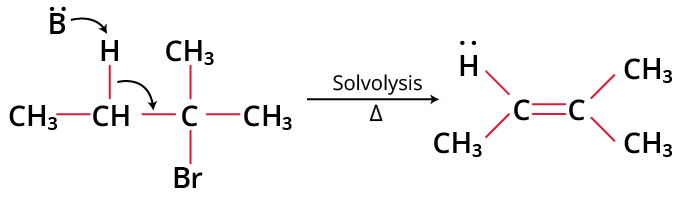
Example of E2 Reaction
E1 Reaction
E1 stands for unimolecular elimination. The reaction is said to be of first order, when the rate of an elimination reaction depends only on the concentration of the substrate and it is designated as E1. E1 is like SN1 reactions. which are also two-step processes.
Step 1: The alkyl halide ionises to give the carbonium ion.
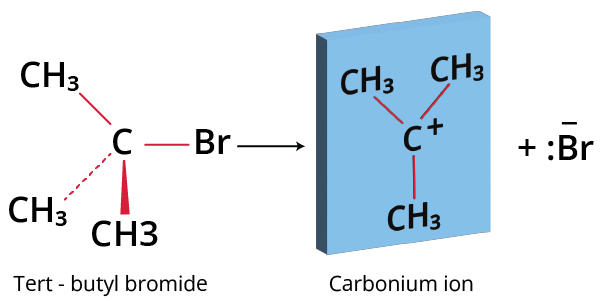
t-butyl bromide to carbonium ion
Step 2: A proton is abstracted by the base from the adjacent carbon atom, which is in the beta position to give the alkene.
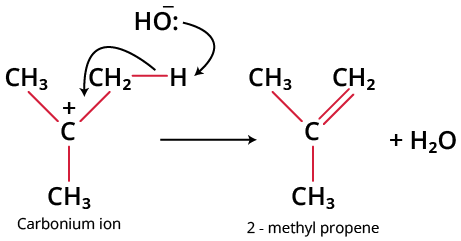
Carbonium ion to 2-methyl propene
If the dehydrohalogenation of an alkyl halide can yield more than one alkene, then according to Saytzeff’s rule, the main product is the most highly substituted alkene. For example, when 2-bromobutane is heated with alcoholic KOH, the number of possible alkenes is 2.
According to the Saytzeff Rule, the main product must be a disubstituted alkene that is 2-butene rather than the monosubstituted alkene, 1 – butene.
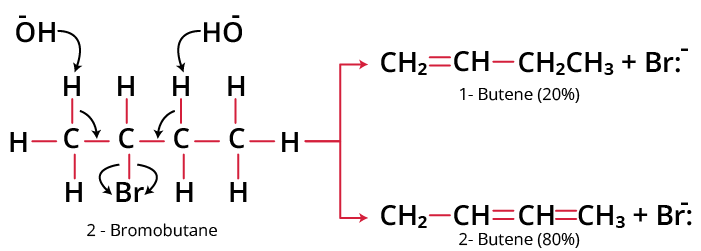
Product of the Saytzeff Reaction
E1 Reaction Mechanism
In ethanol, an equilibrium occurs between the solvent and potassium hydroxide to produce potassium chloride.
$\underset{\text{Ethanol}}{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COOH}}+\mathrm{KOH} \rightleftharpoons \underset{\text{Pot. Ethoxide}}{\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{O}^- \mathrm{K}^{+}}+\mathrm{H}_{2} \mathrm{O}$
Potassium Ethoxide is a strong base. It favours elimination and substitution reactions. There will always be a competition between both elimination and substitution reactions. For example, we can consider the treatment of ethyl bromide with alcoholic KOH that yields either ethylene or diethyl ether. The attacking nucleophile is evidently CH3CH2O:--
Elimination:
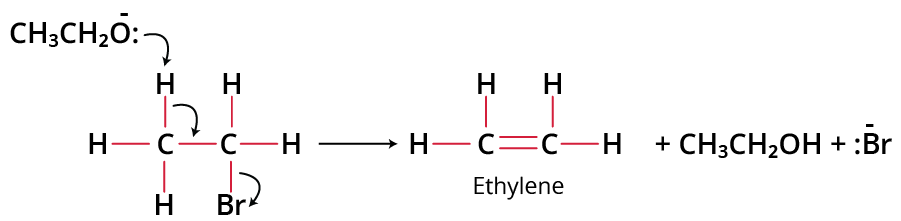
Elimination of Ethylene from Ethyl bromide
Substitution:

Substitution of ethoxide group on Ethyl bromide
The ratio of the elimination to substitution product depends on the structure of the alkyl halide and experimental conditions. Primary and secondary alkyl halides undergo dehydrohalogenation only by the E2 mechanism. Tertiary alkyl halides do so by E1 mechanism.
Let us understand the concept of the mechanism more by considering a more general example.
Step 1: Formation of the Carbocation
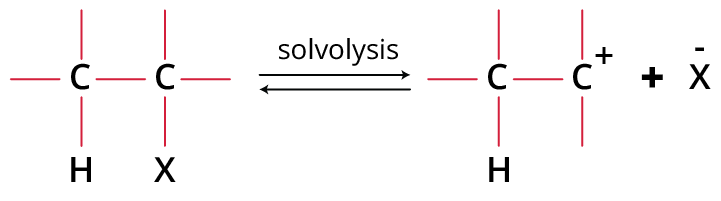
Formation of Carbocation
Step 2: Base abstracts a proton (fast)
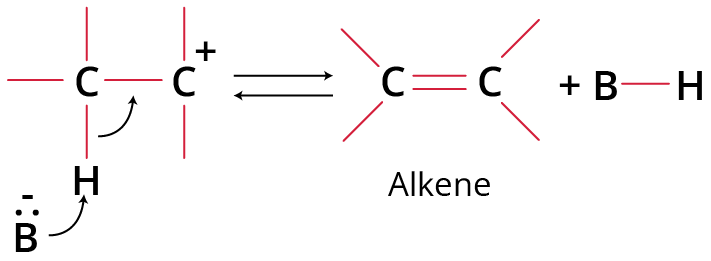
Protonation of Base to form alkene
Substrates capable of giving stable carbocations undergo elimination through the E1 mechanism.
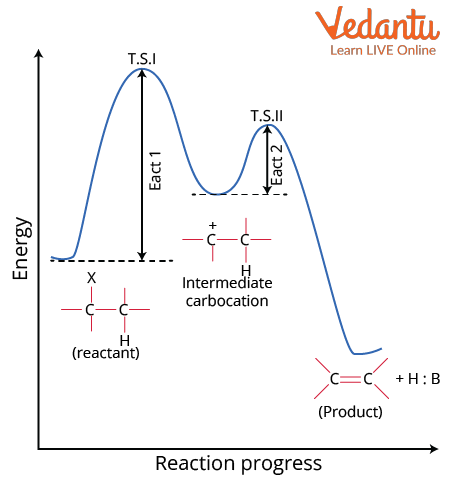
Reaction progress of E1
For example, t-alcohol when heated with concentrated H2SO4 gives isobutylene.
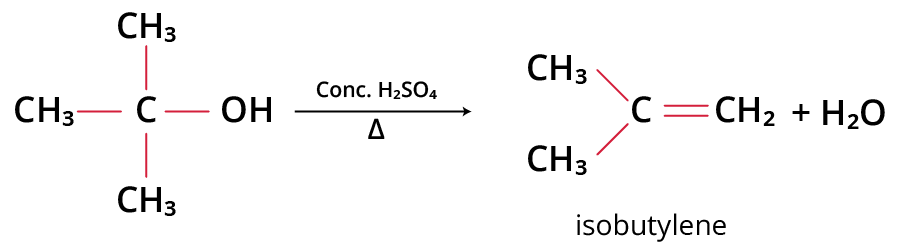
Reaction between t-alcohol and sulphuric acid
E1 elimination is regioselective. It gives Saytzeff products.
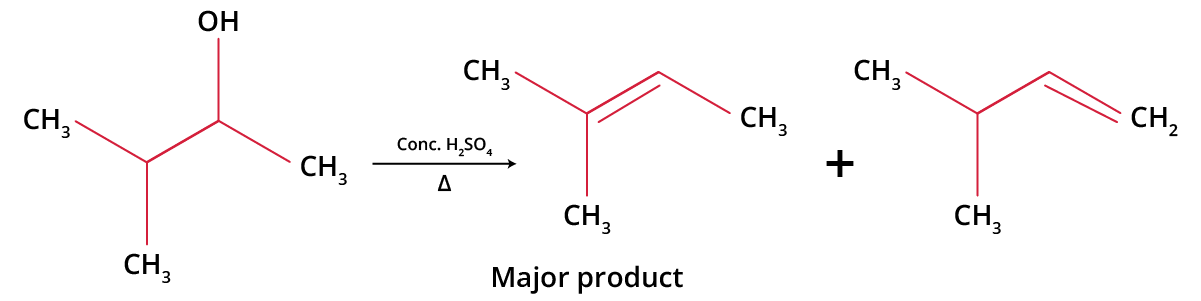
Saytzeff Reaction of t-alcohol
This is mostly true. But there is an exception i.e., vapours of alcohol when passed over ThO2 at 300 – 400o C give Hoffman elimination.
Since carbocation participates, rearrangement is very common in E1 as in SN1. In fact, elimination with change in carbon skeleton is a guaranteed E1 process, though all E1 processes do not necessarily accompany rearrangement. For example:
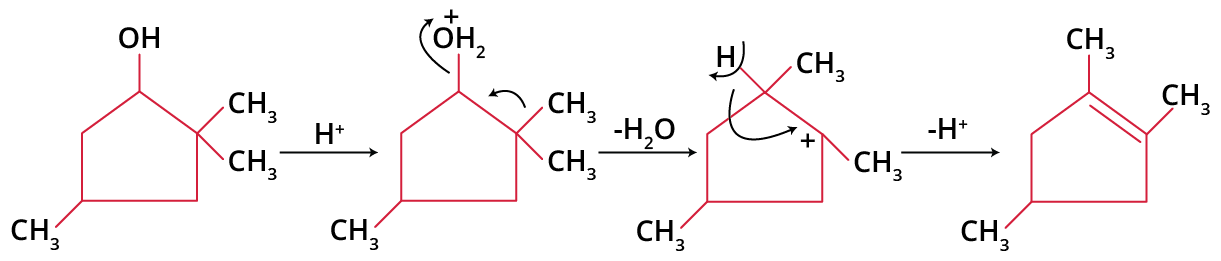
Carbocation rearrangement
In some cases even a single C – C bond migrates during elimination.
C - C bond migration during elimination
Characteristics of E1 Reaction
It is unimolecular, two step process.
It is a first order reaction.
The Reaction intermediate of E1 reaction is carbocation, so there is a possibility of rearrangement.
In the second step of the E1 mechanism, a base abstracts a proton from the carbon atom that is adjacent to the carbocation and forms alkene.
Kinetics: Rate $\propto [alkyl halide]$ Rate = $k[Alkyl halide]$
Examples of E1 Reaction
n-Propyl bromide when put forth for reaction with alcoholic KOH yields propene. In this reaction hydrogen and Bromide are eliminated.
$\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{Br} \stackrel{\mathrm{Alc} \mathrm{KOH}}{\longrightarrow} \mathrm{CH}_{3}-\mathrm{CH}=\mathrm{CH}_{2}+\mathrm{H}_{2} \mathrm{O}+\mathrm{Br}^{-}$
In this reaction, water and bromide are eliminated.
$\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{Br} \stackrel{\mathrm{Alc} \mathrm{KOH}}{\rightarrow}\underset{2-\text{methyl propene}}{\left(\mathrm{CH}_{3}\right)_{2} \mathrm{C}=\mathrm{CH}_{2}}+\mathrm{H}_{2} \mathrm{O}+\mathrm{Br}^{-}$
In the presence of sulfuric acid 2-propanol loses a molecule of water to form propene which is an alkene.
$\mathrm{C}_{3} \mathrm{H}_{7} \mathrm{OH} \rightarrow \mathrm{C}_{3} \mathrm{H}_{6}+\mathrm{H}_{2} \mathrm{O}$
In the presence of methanol, 2-Bromo-3-methyl butane transforms into a combination of 2-methyl-2-butene and 2-methyl-3-butene with the elimination of Hydrogen Bromide.
$\mathrm{C}_{5} \mathrm{H}_{11} \mathrm{Br} \rightarrow \mathrm{C}_{5} \mathrm{H}_{10}+\mathrm{HBr}$
Conclusion
Those organic compounds in which an sp3 hybridised carbon is bonded to an electronegative atom or group of atoms can undergo two types of reactions. Substitution reactions in which the electronegative atom or group is replaced by another atom or group of atoms.
Elimination reaction in which the electronegative atom or groups is eliminated along with hydrogen from an adjacent carbon. The electronegative atom or group which is substituted or eliminated is known as the leaving group. It has partial negative charge and partial charge develops on carbon atoms due to more electronegativity of halogen atoms. As a result of this polar carbon-halogen bond, alkyl halides show nucleophilic substitution and elimination reactions.
FAQs on E1 Reaction - Types, Mechanism and Examples for JEE
1. What are Addition reactions?
Addition reactions are the characteristic reaction of an unsaturated compound (compounds containing a C–C localised double or triple bond). In this type of reaction, two molecules combine to produce a single product. Like substitution, this reaction can also be classified as nucleophilic and free radical addition reactions depending on the type of reagent that initiates the reaction. During the addition reaction, the hybridisation of the substrate changes from sp2 to sp3 in the addition reaction of alkenes or sp to sp2 in the addition reaction of alkynes as only one bond breaks and two new bonds are formed.
2. What are the characteristics of E2 Reaction?
The characteristics of E2 reaction include,
This is a single step bimolecular reaction.
It is a second order reaction.
Kinetics: Rate $\propto [R – X] [Base]$ Rate = k$[R – X]$ $[Base]$
Rearrangement is not possible.
For the lower energy of activation, the transition state must be more stable.
E2 follows a concerted mechanism.
The orientation of the proton and leaving group should be antiperiplanar.
Position of orientation of elimination: In most E1 and E2 eliminations, where there are two or more possible elimination products, the product with the most highly substituted double bond will predominate. This rule is called the saytzeff or zaitsev rule.
























