




Introduction to Fischer Projection
The difficulty of depicting three-dimensional molecules on a two-dimensional surface was first addressed by the Fischer projection. Imagine a light source casting a shadow on a flat surface as it illuminates a three-dimensional item. We can deduce the 3-D structure of the object from the shadow if these things are always placed in the same way. That is what Fischer was aiming for back when dashes, wedges, and CIP rules weren't in use.
R and S System of Nomenclature
A naming scheme known as the R/S system of nomenclature is employed to give chiral compounds an absolute configuration. Latin letters R and S stand for right and left, respectively. A chiral molecule's branches are given priority numbers, and in decreasing order, molecules are either referred to as R or S configured.
When the priority groups are in clockwise order, then the configuration is R, whereas when the priority groups are in anticlockwise order, then the configuration is S.
Example of ( R ) Nomenclature
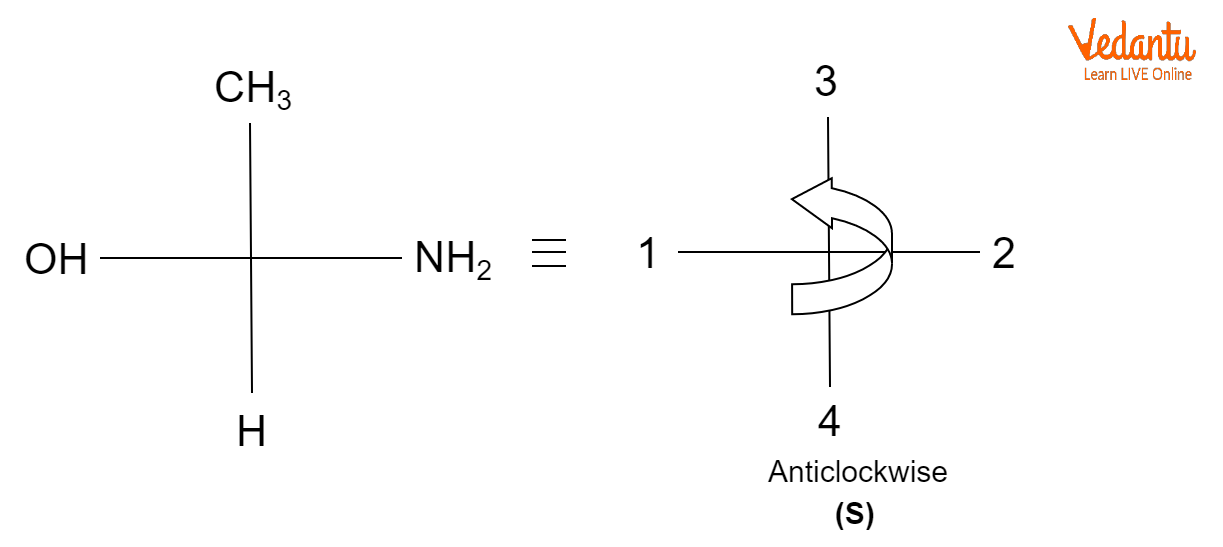
Example of ( S ) Nomenclature
Fischer Projection and Chemical Formula
For large compounds with numerous stereocenters, the wedge and dash representations of stereochemistry can frequently become complicated. The Fischer Projection, invented by the German chemist Emil Fischer, is a different approach to visualising stereochemistry. Every stereocenter is depicted as a cross by the Fischer Projection. Bonds that extend into the plane of the page are represented by the vertical line, while those that extend out of the plane of the page are represented by the horizontal line.
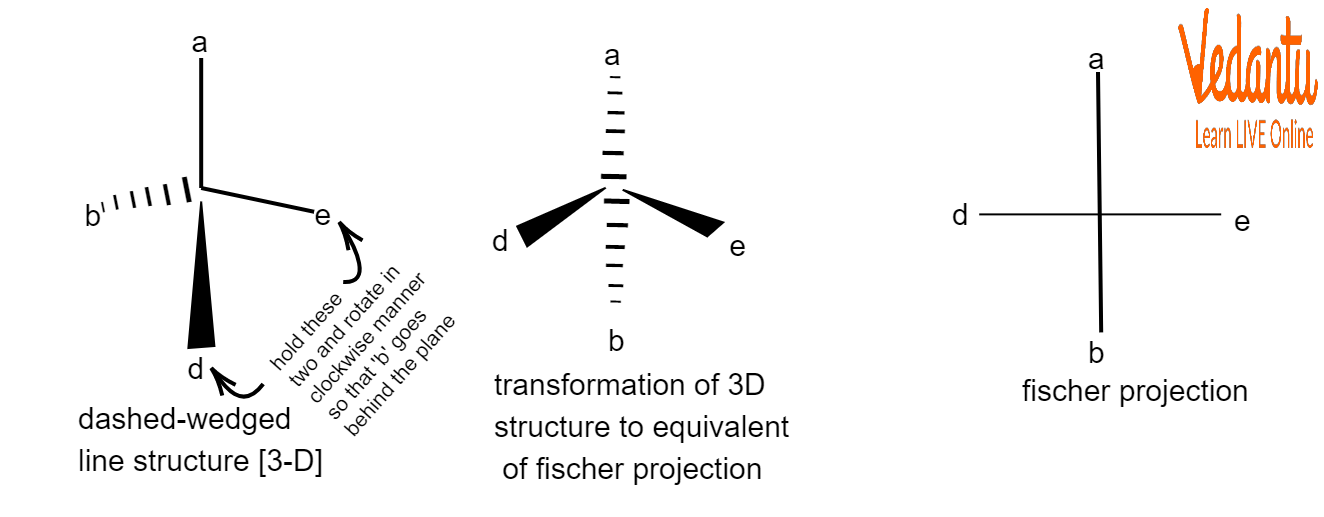
A stereo formula can be represented in two dimensions using the Fischer projection formula without erasing the stereochemical data, or absolute configurations, at chiral centres.
The molecule must be held with the chiral centre on the paper's surface. The two bonds that protrude from the paper's plane ought to be on a horizontal plane. The two bonds that are still in the paper's plane are on a vertical plane.
Example of Fischer Projection
Fischer Projections Rules
A Fischer projection can be turned by 180 degrees without losing its significance since the "up" and "down" portions of the bonds remain constant.
It is forbidden to rotate a Fischer projection by 90 degrees. Usually, such a rotation modifies the enantiomer's conformation.
Simply swap the right and left horizontal linkages in a molecule represented as a Fischer projection to determine its enantiomer.
Draw a horizontal line through the middle of the molecule and check to see if it is symmetric about the line to determine whether the molecule is a meso compound in Fischer projection.
A vertical line is used to symbolise the carbon atoms in a chain, while horizontal and vertical lines are used to indicate the non-terminal atoms. The junction of the two lines will show the carbon atom in the middle even though the carbon atoms may not be represented by the letter itself.
Towards a Fischer projection, all of the horizontal lines are directed in the direction of the viewers, while all of the vertical lines are directed away from the viewers.
All hydrogen atoms must be properly depicted in accordance with IUPAC regulations, especially those that are at the end of the group in a carbohydrate.
Fischer Projection Examples
Monosaccharides and amino acids are typically represented using Fischer projections. They have a lot of stereocenters or carbons with four different bonds, which makes them appropriate for portraying monosaccharides. All monosaccharides are oriented differently along the stereocenter but are generally comparable. We can plainly see the orientation of each monosaccharide thanks to the Fischer projection.
Uses of Fischer Projection
The most typical applications of Fischer projections are in organic Chemistry and biochemistry. A Fischer projection can also be used to distinguish between L- and D- molecules. For instance, by definition, in a Fischer projection, the penultimate carbon of D-sugars is with hydrogen on the left and hydroxyl on the right. The hydrogen atom will be on the right and the hydroxyl atom will be on the left when displaying the L sugars. Drawings are unclear when confused with other types of illustration because Fischer projections in non-carbohydrates are avoided.
Fischer Projection of Lactic Acid
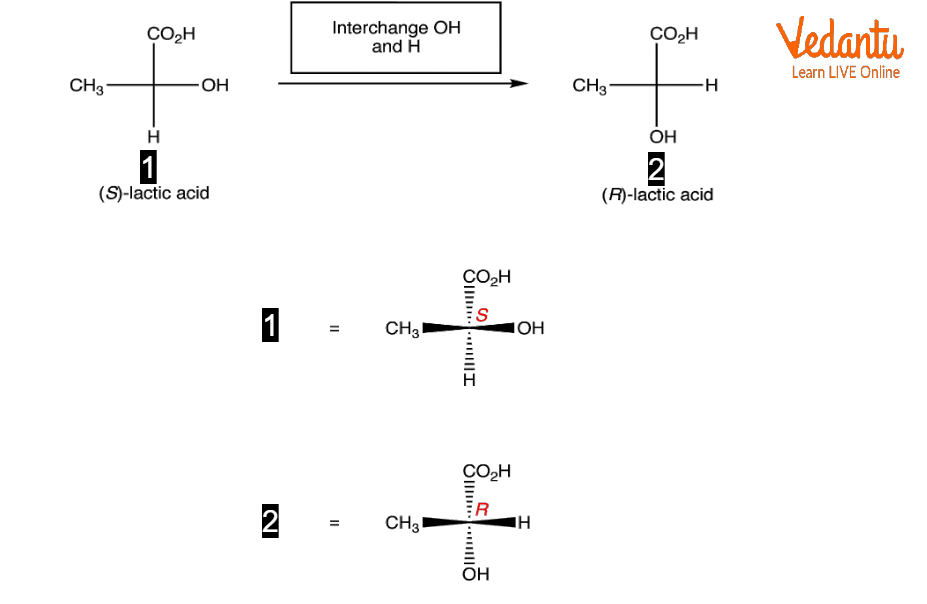
Lactic Acid Fischer Projection
Conclusion
One method for rendering three-dimensional chemical molecules on paper is the Fischer projection. The junction of a horizontal and a vertical line depicts the core carbon atom, while horizontal and vertical lines are utilised to indicate the bonds. The most typical examples of Fischer projection in Organic Chemistry are isomers of various sugars. A naming scheme known as the R/S system of nomenclature is employed to give chiral compounds an absolute configuration.
FAQs on Fischer Projections with R and S Configuration for JEE
1. How to determine R/S configuration when there is a tie?
The plane of polarised light rotated by one of a pair of R or S structured compounds in a solution is cancelled by the other. Because a shift of plane can only occur if the concentration of both types of chemicals is precisely equal, this implies that there is no such shift. A racemic mixture is the name given to this class of tie-in solutions.
2. What is the difference between Fischer projection and Haworth structure?
The Fischer projection depicts the open chain structure of organic molecules, whereas the Haworth projection depicts the closed-cyclic structure of organic molecules. This is the main distinction between the two projections. The molecular structure of organic molecules can be displayed using the Fischer projection or the Haworth projection. Fischer projection uses projection to depict organic molecules in two dimensions, while Haworth projection uses a three-dimensional perspective to depict the cyclic structure of monosaccharides.

























