




Haber's Process for Manufacture of Ammonia
Fritz Haber in 1909 found the conditions under which Nitrogen (N2) and Hydrogen (H2) can combine to form ammonia ( NH3), which yields upto 10-20% approximately.
The conditions are as follows:
Very high pressure
Low temperature
Porous iron catalyst.
Industrial Process: Carl Bosch developed the Haber’s process into the industries.
The Haber’s process is an exothermic reaction releasing upto 92.4kJ/mol of energy at 298K (25 degree Celcius).
Haber’s process reaction is as follows:
$\mathrm{N}_{2}(\mathrm{~g})+\mathrm{H}_{2}(\mathrm{~g}) \xrightarrow{\text { iron catalyst, } 400^{\circ} \mathrm{C}, 200 \mathrm{~atm}}\mathrm{NH}_{3}(\mathrm{~g})$
We will learn about it, the process for the manufacture of ammonia, the reactions involved in the process, the related diagram, and the conditions necessary for carrying out the Haber’s process.
Haber’s Process
In ammonia preparation by Haber’s process, first the nitrogen gas is taken from the air which is then combined with the hydrogen atom that is extracted from the natural gas in the ratio 1:3 by volume in the reactor. According to the Avagadro’s law, at same temperature and pressure, equal number of gases contains equal number of molecules.
The gases then pass through the beds of the catalyst where cooling takes place which maintains the equilibrium constant. During the passage of the gases different forms of conversion take place and the unreacted gases are recycled. Iron is used as a catalyst and a temperature of 400-500 degree Celcius and pressure of 150-200 atm is maintained.
Through continuous recycling of unreacted gases like nitrogen and hydrogen, around 15% of nitrogen and hydrogen is transformed into ammonia (this may vary from plant to plant), and we get results of about 98 percent. Finally, the generated ammonia is cooled to form a liquid solution that is collected and stored in containers.
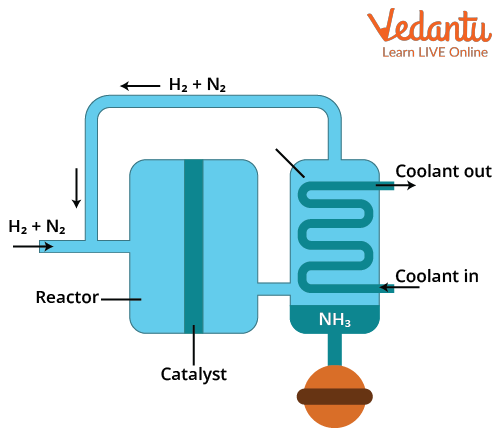
Haber’s process for the manufacture of ammonia
Reaction of Haber’s Process
$\underset{ \text{nitrogen}}{\mathrm{N}_{2}(\mathrm{~g})}+\underset{\text { hydrogen }}{3 \mathrm{H}_{2}(\mathrm{~g})} \xrightarrow{\text { heat, pressure, catalyst }}\underset{\text { ammonia }}{2 \mathrm{NH}_{3}(\mathrm{~g})}+ (-92.4 \mathrm{~kJ} \mathrm{~mol}^{-1}) $
OR
$\underset{\text{nitrogen}}{\mathrm{N}_{2}(\mathrm{~g})}+\underset{\text{hydrogen}}{3 \mathrm{H}_{2}(\mathrm{~g})} \xrightarrow{\text { heat, pressure,catalyst }}\underset{\text { ammonia }}{2 \mathrm{NH}_{3}(\mathrm{~g})}+92.4 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Haber Process Catalyst
Haber's technique uses a metal as a catalyst. In most cases, iron is used as a catalyst in this process. Iron is favoured since it allows for a higher production of a product in a shorter amount of time. Promoters such CaO, K2O, SiO2, and Al2O3 are sometimes used. Osmium was employed as a catalyst in the past. Iron, on the other hand, is a low-cost catalyst that is still utilised today.
Effect on the equilibrium of Haber’s process upon using the catalyst: The catalyst has no effect on the equilibrium position. Adding a catalyst to the equilibrium mixture does not result in a higher amount of ammonia in the mixture. Its sole purpose is to accelerate the reaction.
Effect on the rate of Haber’s process upon using the catalyst: Without a catalyst, the reaction is so slow that almost no reaction occurs in a reasonable amount of time. The catalyst guarantees that the reaction proceeds quickly enough for a dynamic equilibrium to form in the brief time that the gases are in the reactor.
Haber Process Diagram
The ratio of the nitrogen and hydrogen gases is introduced into the reacting cylinder. The iron catalyst is added, and the temperature and pressure are regulated. Ammonia gas is produced as a result of the reaction. This ammonia gas is compressed and cooled, turning it from a gas to a liquid at the last ammonia liquid is separated.
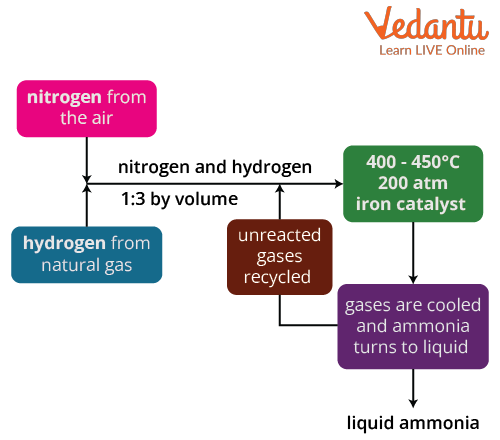
Block diagram of Haber’s process
Conditions for Haber’s Process
The following are the essential conditions for the creation of ammonia in Haber’s process are as follows;
1) Low temperature: Ammonia is formed through an exothermic process. As a result, the creation of ammonia is favoured by low temperatures.
2) High pressure: Because the number of product molecules is less than the number of reactant molecules. As a result, high pressure encourages ammonia production.
3) Presence of catalyst: Ammonia is formed in the presence of a catalyst and a promoter, namely Fe and Mo.
Conclusion
Synthesis of ammonia by Haber's process is used to produce ammonia on a large scale. The components of ammonia (N2) and hydrogen (H2) mix in a 1:3 ratio.
$\mathrm{N}_{2}+3 \mathrm{H}_{2} \rightleftharpoons 2 \mathrm{NH}_{2}$
The reaction is exothermic because it progresses in a forward direction with a significant decrease in volume. High pressure would favour the creation of ammonia, according to Le Chatelier's principle. The ideal circumstances for the manufacture of ammonia include a pressure of approximately 200 atm, a temperature of approximately 700K, and the employment of a catalyst such as iron oxide with minor amounts of K2O and Al2O3 to speed up the rate of equilibrium attainment.
FAQs on Haber's Process for Manufacture of Ammonia for JEE
1. What is Le Chatelier's principle?
Henri Le Chatelier, a French chemist, proposed in 1884 when an equilibrium system is disturbed, the equilibrium position shifts in the direction that tends to minimise the disturbance's influence. Chatelier's principle has several crucial implications for a reversible chemical reaction, as shown below:
When the concentration of a reactant is raised, the equilibrium position shifts to produce more products in order to utilise the change.
When the pressure on this equilibrium system is increased, the equilibrium position shifts to the side with the fewest gas particles, i.e. to the side with the least number of gas particles.
2. What are the uses of ammonia in everyday life?
Ammonia has the following uses in everyday life-
Fertiliser: More than 80% of the synthetic anhydrous ammonia is used as an agricultural fertiliser. The released nitrogen helps for plants i.e. crops to thrive by providing essential nutrients such as zinc, selenium and boron.
Cleaning agent: Ammonium hydroxide, also known as household ammonia, is widely used in cleaning household products such as tubs, tiles, sinks and so on. Because ammonia evaporates quickly, it is also useful for cleaning greasy stains on glass.
Industries: It is used to clean water supplies and manufacture of many products like explosives, pesticides, and dyes.

































