




How Does the Inductive Effect Affect the Acidity of Organic Compounds?
Understanding the Inductive Effect and Acidic Strength is crucial for JEE Main Organic Chemistry. The inductive effect describes how electron density is transmitted through sigma bonds when atoms or groups with different electronegativities are present, directly impacting properties such as acidity. Accurate application of this principle allows students to compare the acidic strength of organic compounds and predict trends in acidity and pKa values in exam problems.
What is Inductive Effect?
The inductive effect refers to the permanent shifting of bonding electrons towards a more electronegative atom in a molecule, creating a partial charge along the carbon chain. This electronic effect only acts through single (sigma) bonds and weakens with distance from the source group. An everyday example is when a chlorine atom (more electronegative than carbon) pulls electron density towards itself in chloroethane, making the adjacent carbon partially positive.

Types of Inductive Effect: +I and –I with Examples
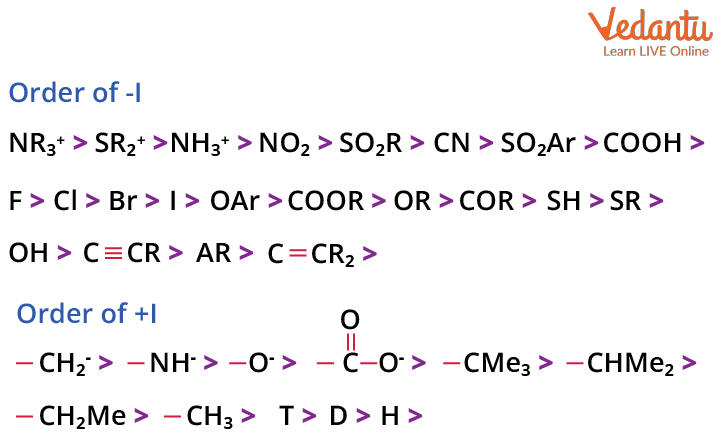
There are two types of inductive effects based on whether a group donates or withdraws electrons through sigma bonds. These are the +I (positive inductive) effect and the –I (negative inductive) effect. Recognising these enables you to predict how substituent groups will influence molecular stability and reactivity.
| Effect | Definition | Typical Groups | Examples |
|---|---|---|---|
| –I (Electron-withdrawing) | Pulls electrons away; increases δ+ near group | –NO2, –Cl, –CN, –COOH | Cl–CH2–COOH, NO2–C6H4–COOH |
| +I (Electron-donating) | Pushes electrons towards rest of molecule | –CH3, –C2H5, –(CH2)n– | (CH3)3N–CH2–COOH |
Order and Range of the Inductive Effect
The magnitude of the inductive effect depends on how close a substituent is to the main functional group, such as the carboxyl group in carboxylic acids. The effect is strongest at the atom directly attached and fades rapidly along more carbon atoms. For example, in chloropropanoic acids, a chlorine atom at the alpha position (next to –COOH) increases the effect, but its influence drops with each additional carbon.
Inductive Effect and Acidic Strength: The Key Relationship
The primary application for JEE is using the inductive effect to predict acidic strength. Acidic strength relates to the ability of a molecule (usually an acid, HA) to donate a proton (H+). After proton loss, the stability of the resulting conjugate base (A–) is essential. Electron-withdrawing (–I) groups stabilise the negative charge on A– by pulling electron density away, increasing acidity. Electron-donating (+I) groups destabilise the anion, reducing acid strength.
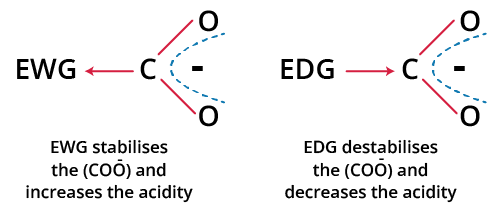
For example, comparing acetic acid (CH3COOH) and chloroacetic acid (ClCH2COOH): the –Cl group shows a strong –I effect, resulting in a much stronger acid (lower pKa) than an acid with a +I group like –CH3.
Formula Guide: Predicting Acidic Strength Using the Inductive Effect
Key principle: Acidity ∝ Stability of Conjugate Base. The more stable the anion by inductive withdrawal, the stronger the acid.
- Stronger –I groups → higher acidity (lower pKa)
- More +I groups → lower acidity (higher pKa)
- Multiple –I substituents → significant increase in acidity
- Inductive effect falls drastically with greater distance from –COOH
Acidic strength order for carboxylic acids (in presence of –I groups):
ClCH2COOH > CH3COOH > C2H5COOH
Exam-Style Example: Apply Inductive Effect and Acidic Strength
Question: Arrange these in increasing acidic strength: CH3COOH, ClCH2COOH, Cl2CHCOOH.
Solution: More chlorine means more –I effect stabilising the conjugate base. Therefore, CH3COOH < ClCH2COOH < Cl2CHCOOH (strongest acid, lowest pKa). Always check for number and position of electron-withdrawing groups.
Quick Notes, Common Pitfalls, and Memory Aids
- pKa falls as acid strength rises (inverse relation!).
- –I effect outweighs +I for acidity; position matters greatly.
- Don’t confuse with resonance: resonance delocalises negative charge differently.
- Inductive effect operates only through sigma bonds, not via pi bonds.
- For JEE MCQs, memorise order: –NO2 > –CN > –F > –Cl > –Br > –I (decreasing –I effect).
- Distance trap: the further the substituent, the weaker its effect on acidity.
Contrast: Inductive Effect vs Resonance in Acid-Base Chemistry
| Feature | Inductive Effect | Resonance Effect |
|---|---|---|
| Bond type | Sigma only | Pi (delocalisation) |
| Distance dependency | Falls rapidly | Similar across the system |
| Dominates for | Halogens, alkyls | Conjugated compounds |
| Key effect seen in | Acidic strength order in haloacids | Phenols, carboxylates |
Practice Problems for JEE Main Mastery
- Arrange in order of increasing acidity: FCH2COOH, ClCH2COOH, BrCH2COOH, ICH2COOH.
- Which acid is stronger and why? (CH3)2CHCOOH or CH3COOH?
- If –NO2 is placed meta to –COOH, will acidity rise as much as in ortho? Justify.
- State one reason why –I effect decreases quickly in longer carbon chains.
- Does the inductive effect explain acidity ordering in aromatic carboxylic acids? Give a brief reason.
For stepwise solutions and more JEE Chemistry short notes, Vedantu's curated question sets help reinforce both fundamental and tricky points.
Interconnections and Related JEE Main Chemistry Concepts
- Review Resonance Effect to see effects beyond induction.
- Solidify acid-base patterns with Acids, Bases, and Salts.
- Explore Halogen Effects in Organic Compounds for real inductive examples.
- Dive into Inductive vs Resonance Effect for final revision.
- Grasp overall Chemical Bonding and Molecular Structure basics for context.
- Master Important Questions on Organic Halogens to encounter more –I/EDG MCQs.
- Clarify any confusion on General Organic Chemistry Principles.
- Use Hydrogen Bonding for tricky exceptions in acidity trends.
- Turn to Oxygen-Containing Compounds for further practice with acids and ketones.
In summary, the Inductive Effect and Acidic Strength topic forms the core of JEE Main Organic Chemistry reasoning. By combining inductive reasoning, group effects, and the logic of conjugate base stabilisation, you can answer most acid strength MCQs confidently. For more systematic practice and topic-wise micro-revision, consult Vedantu's tailored JEE Chemistry resources.
FAQs on Inductive Effect and Its Role in Acidic Strength
1. How does the inductive effect affect acidic strength?
The inductive effect influences acidic strength by shifting electron density through sigma bonds, affecting how easily an acid loses a proton (H+).
Key points include:
- Electron-withdrawing groups (−I effect) attached to an acid pull electrons away, increasing acid strength by stabilizing the negative charge on the conjugate base.
- Electron-donating groups (+I effect) push electrons toward the acidic center, decreasing acid strength by destabilizing the conjugate base.
- More inductive withdrawal usually means a lower pKa and a stronger acid.
2. Does +I effect increase acidity?
No, the +I effect generally decreases acidity in organic compounds.
Here’s how:
- Electron-donating groups (such as alkyl chains) exhibit +I effect, pushing electron density toward the acidic hydrogen.
- This makes it harder to lose H+, lowering the acidic strength.
- In exam questions, compounds with more +I groups are often weaker acids.
3. Does inductive effect increase pKa?
The impact of the inductive effect on pKa depends on whether groups are electron-withdrawing or donating.
- Electron-withdrawing (−I) groups decrease pKa (acidity increases).
- Electron-donating (+I) groups increase pKa (acidity decreases).
4. Does inductive electron withdrawal increase acidity?
Yes, inductive electron withdrawal by electronegative groups increases acidity.
Key effects:
- Pulls electron density away from the acidic site.
- Stabilizes the conjugate base after deprotonation.
- Results in a stronger acid (lower pKa).
5. What is the difference between inductive effect and acidic strength?
The inductive effect is a phenomenon, while acidic strength is an outcome it can influence.
- Inductive effect: Electron shift through sigma bonds due to attached groups or atoms.
- Acidic strength: Tendency of a substance to donate H+.
- The inductive effect modifies acidic strength by stabilizing/destabilizing the conjugate base.
6. Can resonance sometimes override the inductive effect in determining acidity?
Yes, resonance can be a stronger effect than induction for acidity trends.
- Resonance delocalizes charge over more atoms, often stabilizing the conjugate base more than inductive effects alone.
- When both are possible, resonance usually dominates unless interrupted by specific group locations.
7. Are all electron-withdrawing groups equally effective in increasing acidity?
No, electron-withdrawing groups (−I effect) differ in their ability to enhance acidity.
Effectiveness depends on:
- Electronegativity of the atom/group
- Number and proximity of such groups to the acidic site
- Molecule structure (straight chain vs. branched, etc.)
8. How does the inductive effect operate in polyfunctional compounds?
In polyfunctional compounds, the inductive effect is additive and depends on the number, type, and position of each substituent.
- Multiple electron-withdrawing groups can significantly increase acidic strength.
- Electron-donating and withdrawing groups can compete, affecting overall acidity.
- The effect diminishes with distance from the acidic hydrogen.
9. Does distance from the acidic hydrogen affect inductive influence?
Yes, the inductive effect weakens with increasing distance from the acidic hydrogen.
- Groups closer to the acid site exert a stronger influence.
- Substituents further away (beyond 2-3 carbons) have a much smaller effect on acidic strength.
10. Can inductive effect explain the acidity trend in carboxylic acid derivatives?
Yes, inductive effect helps explain why some carboxylic acid derivatives are more acidic than others.
- Electron-withdrawing groups attached to the alpha carbon or within the functional group increase acidity (lower pKa).
- Electron-donating groups do the reverse.
- This trend is commonly asked in JEE Main/NEET MCQs involving chloroacetic acid, nitroacetic acid, and methylacetic acid.
































