
Why does \[N{H_3}\] act as a Lewis base?
Answer
233.1k+ views
Hint : A Lewis base is a substance that can donate a pair of non bonding electrons. A Lewis base is therefore called electron-pair-donor. In Lewis base molecules the orbitals used to be highly occupied so they have a tendency to donate electrons. The most common Lewis base is ammonia .
Complete answer:
> In ammonia the molecule consists of a nitrogen atom as a central metal atom. Ammonia is Lewis base because nitrogen has a lone pair of electrons which can be donated, hence it acts as Lewis base. \[N{H_3}\] can give a lone pair of electrons to protons to form ammonium ions . This reaction shows its Lewis base tendency. $N{H_3} + {H^ + } \to N{H^ + }_4$ $ \Rightarrow $ Here in this reaction the lone pair on the nitrogen atom is transferred to the hydrogen ion . Hydronium ion acts as a Lewis acid here which accepts electrons from ammonia.
> A Lewis acid-base reaction can be done when a Lewis base donates a pair of electrons to an Lewis acid. Lets see an example of Lewis acid-base reaction. In the following reaction each of two ammonia molecules which is a Lewis base donates a pair of electrons to a silver ion which acts as Lewis acid in the reaction.
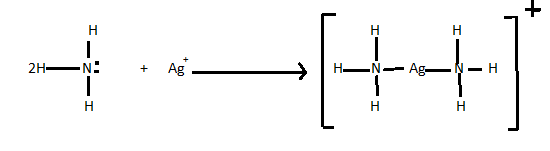 There are so many reactions that are Lewis acid-base displacement reactions.
There are so many reactions that are Lewis acid-base displacement reactions.
Note : Here we learned about Lewis base and also learned why ammonia acts as a Lewis base in any reaction. Ammonia has a filled orbital containing an electron pair which does not involve bonding and Lewis acid contains an empty orbital which is capable of accepting electrons.
Complete answer:
> In ammonia the molecule consists of a nitrogen atom as a central metal atom. Ammonia is Lewis base because nitrogen has a lone pair of electrons which can be donated, hence it acts as Lewis base. \[N{H_3}\] can give a lone pair of electrons to protons to form ammonium ions . This reaction shows its Lewis base tendency. $N{H_3} + {H^ + } \to N{H^ + }_4$ $ \Rightarrow $ Here in this reaction the lone pair on the nitrogen atom is transferred to the hydrogen ion . Hydronium ion acts as a Lewis acid here which accepts electrons from ammonia.
> A Lewis acid-base reaction can be done when a Lewis base donates a pair of electrons to an Lewis acid. Lets see an example of Lewis acid-base reaction. In the following reaction each of two ammonia molecules which is a Lewis base donates a pair of electrons to a silver ion which acts as Lewis acid in the reaction.
 There are so many reactions that are Lewis acid-base displacement reactions.
There are so many reactions that are Lewis acid-base displacement reactions.Note : Here we learned about Lewis base and also learned why ammonia acts as a Lewis base in any reaction. Ammonia has a filled orbital containing an electron pair which does not involve bonding and Lewis acid contains an empty orbital which is capable of accepting electrons.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




