
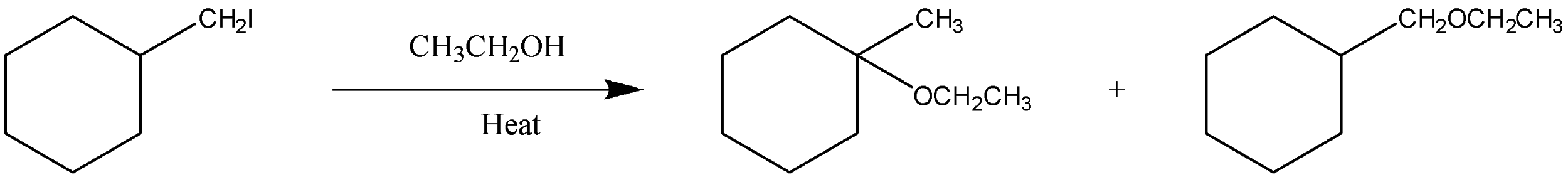
Is the above reaction an example of solvolysis reaction? Give the reason for your answer.
Answer
233.1k+ views
Hint: Solvolysis reactions are kind of substitution reactions. In these reactions solvent displaces an atom present in a molecule. The ethanol given here is a solvent molecule.
Complete step by step answer:
* First, let us know about the solvolysis, or solvolytic reactions. In this reaction an atom, or group present in a molecule is replaced by another atom, or group of atoms. The solvent present in the reaction produces electron-rich atoms that act as a nucleophile, and displace an atom.
* Now, in the given reaction ethanol present behaves as a solvent as mentioned, so it will act as a nucleophile too.
* The first step will be the formation of a carbocation by displacement of an atom I present at the ortho position, we can see it in the figure.
* In the second step, nucleophile formed will attack, and then there will be the deprotonation of molecules.
Thus, we can say that the end product given in the figure will be formed after the deprotonation of a molecule.
So, this mechanism, and the reaction given satisfies all the conditions of solvolysis reaction.
Hence, we can conclude that the given reaction is an example of solvolysis reaction.
Note: Don’t get confused while identifying the reaction, we can clearly see that there is presence of ethanol which is a solvent, and an atom is also present to displace, and form a carbocation. So, at last there is removal of a proton. Just do it step by step, and we would know the answer.
Complete step by step answer:
* First, let us know about the solvolysis, or solvolytic reactions. In this reaction an atom, or group present in a molecule is replaced by another atom, or group of atoms. The solvent present in the reaction produces electron-rich atoms that act as a nucleophile, and displace an atom.
* Now, in the given reaction ethanol present behaves as a solvent as mentioned, so it will act as a nucleophile too.
* The first step will be the formation of a carbocation by displacement of an atom I present at the ortho position, we can see it in the figure.
* In the second step, nucleophile formed will attack, and then there will be the deprotonation of molecules.
Thus, we can say that the end product given in the figure will be formed after the deprotonation of a molecule.
So, this mechanism, and the reaction given satisfies all the conditions of solvolysis reaction.
Hence, we can conclude that the given reaction is an example of solvolysis reaction.
Note: Don’t get confused while identifying the reaction, we can clearly see that there is presence of ethanol which is a solvent, and an atom is also present to displace, and form a carbocation. So, at last there is removal of a proton. Just do it step by step, and we would know the answer.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




