
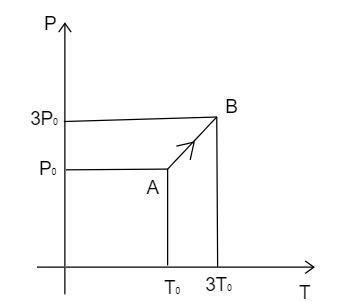
Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point $A$ is ${\rho _0}$ . Density at point $B$ will be:
(A) $\dfrac{3}{4}{\rho _0}$
(B) $\dfrac{3}{2}{\rho _0}$
(C) $\dfrac{4}{3}{\rho _0}$
(D) $2{\rho _0}$
Answer
232.8k+ views
Hint Use the formula of the ideal gas equation, substitute the relation between the volume and the density in it. Solving it provides the value of the density. Find the same for the point $A$ and $B$ by substituting the temperature and the pressure at them.
Useful formul
(1) The formula of the ideal gas equation is given by
$PV = nRT$
Where $P$ is the pressure, $V$ is the volume, $n$ is the number of moles, $R$ is the gas constant and $T$ is the temperature.
(2) The relation between the two volume and the molar mass is given by
$\dfrac{n}{V} = \dfrac{d}{M}$
Where $d$ is the density of the gas and $M$ is the molar mass of the gas.
Complete step by step solution
Observe the diagram and analyze the value of the pressure and the temperature in the point $A$ and $B$ .
Let us write the ideal gas equation,
$PV = nRT$
Bring the volume to the right hand side of the equation, we get
$P = \dfrac{{nRT}}{V}$
Substituting the relation (2) in the above equation, we get
$P = \dfrac{d}{M}RT$
The density of the gas is found as
$d = \dfrac{{PM}}{{RT}}$ ------------(1)
Substituting the value of the temperature and the pressure at a point $A$,
$d = \dfrac{{{P_A}M}}{{R{T_A}}}$
Substituting the values,
${\rho _0} = \dfrac{{{P_0}M}}{{R{T_0}}}$ --------------(2)
Substituting the (1) with the value of the temperature and the pressure at a point $B$ ,
$d = \dfrac{{{P_B}M}}{{R{T_B}}}$
${d_B} = \dfrac{{3{P_0}M}}{{R2{T_0}}} = \dfrac{3}{2}{\rho _0}$ ---------------(3)
Thus the option (B) is correct.
Note Ideal gas equation is also known as the equation of the states, because this equation uses the variables in it to determine or explain about the state of the gas that is considered. This is mainly used to interconvert the volume with the molar mass.
Useful formul
(1) The formula of the ideal gas equation is given by
$PV = nRT$
Where $P$ is the pressure, $V$ is the volume, $n$ is the number of moles, $R$ is the gas constant and $T$ is the temperature.
(2) The relation between the two volume and the molar mass is given by
$\dfrac{n}{V} = \dfrac{d}{M}$
Where $d$ is the density of the gas and $M$ is the molar mass of the gas.
Complete step by step solution
Observe the diagram and analyze the value of the pressure and the temperature in the point $A$ and $B$ .
Let us write the ideal gas equation,
$PV = nRT$
Bring the volume to the right hand side of the equation, we get
$P = \dfrac{{nRT}}{V}$
Substituting the relation (2) in the above equation, we get
$P = \dfrac{d}{M}RT$
The density of the gas is found as
$d = \dfrac{{PM}}{{RT}}$ ------------(1)
Substituting the value of the temperature and the pressure at a point $A$,
$d = \dfrac{{{P_A}M}}{{R{T_A}}}$
Substituting the values,
${\rho _0} = \dfrac{{{P_0}M}}{{R{T_0}}}$ --------------(2)
Substituting the (1) with the value of the temperature and the pressure at a point $B$ ,
$d = \dfrac{{{P_B}M}}{{R{T_B}}}$
${d_B} = \dfrac{{3{P_0}M}}{{R2{T_0}}} = \dfrac{3}{2}{\rho _0}$ ---------------(3)
Thus the option (B) is correct.
Note Ideal gas equation is also known as the equation of the states, because this equation uses the variables in it to determine or explain about the state of the gas that is considered. This is mainly used to interconvert the volume with the molar mass.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




