
The biodegradable polymer nylon-$2$ -nylon-$6$ is formed by condensation of glycine and______.
A. acrylonitrile
B. amino caproic acid
C. alanine
D. adipic acid
Answer
232.8k+ views
Hint: Biodegradable polymers are polymers which can be degraded or broken down by other living organisms like bacteria so that the polymer does not cause any serious effect on the environment.
Step by Step Explanation: Nylon-$2$ -nylon-$6$ is a biodegradable polymer. It is a polyamide and is obtained by condensation of monomers glycine $\left( {{{\text{H}}_2}{\text{N - C}}{{\text{H}}_2}{\text{ - COOH}}} \right)$ and amino caproic acid $\left[ {{{\text{H}}_2}{\text{N}}{{\left( {{\text{C}}{{\text{H}}_2}} \right)}_5}{\text{COOH}}} \right]$ . When n numbers of glycine are condensed with n numbers of amino caproic acid, Nylon-$2$ -nylon-$6$ is obtained with the loss of water molecules. The reaction is as follows:
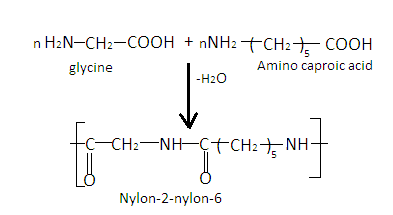
Nylon-$2$ -nylon-$6$ is an alternating polyamide copolymer as it is prepared from two monomers. It is used in-
- Synthesis of artificial fibers.
- As a thread in the bristles of a toothbrush.
- In making strings of musical instruments.
- As matrix material.
- In car components next to the engine due to heat resistance property.
Hence the correct answer is B.
Additional Information: Besides Nylon-$2$ -nylon-$6$, dextran and PHBV which are both polyesters are also bio-degradable polymers which can be degraded by bacteria in the environment. The characteristics of biodegradable polymers are-
1. They are inert and non-toxic
2. They have bio-compatibility.
3. They have permeability.
4. They have tensile strength and mechanical strength.
5. They have controlled the rate of degradation as they can be easily degraded within a suitable time –period.
Note: Biodegradable polymers have many uses in medical field-
1. They are used as orthopedic devices
2. They are used as implants and sutures.
3. They are also used as rug-release matrices.
Step by Step Explanation: Nylon-$2$ -nylon-$6$ is a biodegradable polymer. It is a polyamide and is obtained by condensation of monomers glycine $\left( {{{\text{H}}_2}{\text{N - C}}{{\text{H}}_2}{\text{ - COOH}}} \right)$ and amino caproic acid $\left[ {{{\text{H}}_2}{\text{N}}{{\left( {{\text{C}}{{\text{H}}_2}} \right)}_5}{\text{COOH}}} \right]$ . When n numbers of glycine are condensed with n numbers of amino caproic acid, Nylon-$2$ -nylon-$6$ is obtained with the loss of water molecules. The reaction is as follows:

Nylon-$2$ -nylon-$6$ is an alternating polyamide copolymer as it is prepared from two monomers. It is used in-
- Synthesis of artificial fibers.
- As a thread in the bristles of a toothbrush.
- In making strings of musical instruments.
- As matrix material.
- In car components next to the engine due to heat resistance property.
Hence the correct answer is B.
Additional Information: Besides Nylon-$2$ -nylon-$6$, dextran and PHBV which are both polyesters are also bio-degradable polymers which can be degraded by bacteria in the environment. The characteristics of biodegradable polymers are-
1. They are inert and non-toxic
2. They have bio-compatibility.
3. They have permeability.
4. They have tensile strength and mechanical strength.
5. They have controlled the rate of degradation as they can be easily degraded within a suitable time –period.
Note: Biodegradable polymers have many uses in medical field-
1. They are used as orthopedic devices
2. They are used as implants and sutures.
3. They are also used as rug-release matrices.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




