
The C-C bond length of the following molecules is in the order:
\[
{\text{(A)}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ > }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ > }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ > }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}} \\
{\text{(B)}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} \\
{\text{(C) }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} \\
{\text{(D) }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}} \\
\]
Answer
233.1k+ views
Hint: The carbon-carbon bond lengths are dependent upon the type of bonds namely single bond, double bond, or triple bond. The single bond has more bond length compared to double bond which in turn is more than triple bond.
Complete step by step answer:
Bond lengths decrease with increase in s-character. In other words, multiple bonds have a shorter bond length as compared to a single bond.
In the case of single bond only sigma bonds are present whereas in double bond a sigma and a pi bond are present. Sigma bonds are weaker bonds but have high bond length compared to a pi bond. In triple bonds there are two pi bonds which makes it a shorter bond.
A typical carbon-carbon single bond has a length of 154 pm, while a typical double bond and triple bonds are 134 pm and 120 pm, respectively.
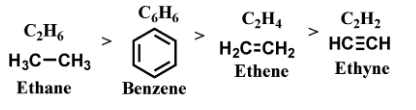
We can see that
Ethane has a single bond between carbon and carbon.
In benzene, the carbon-carbon bond lengths are in resonance due to its aromatic nature, so they have bond length between single bond and double bond as it exhibits partial double bond character.
In ethene, there is a double bond between carbon and carbon.
In ethyne, there is a triple bond between carbon and carbon.
Thus, Single bond > Partial double bond > Double bond > Triple bond.
Therefore, we get the correct following order:
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]
So, the correct option is B.
Note: Partial double bond character is exhibited by molecules having resonance structures where both single bonds and double bonds are exhibited by the molecule. These molecules have bond lengths more than single bonds but less than double bonds.
Complete step by step answer:
Bond lengths decrease with increase in s-character. In other words, multiple bonds have a shorter bond length as compared to a single bond.
In the case of single bond only sigma bonds are present whereas in double bond a sigma and a pi bond are present. Sigma bonds are weaker bonds but have high bond length compared to a pi bond. In triple bonds there are two pi bonds which makes it a shorter bond.
A typical carbon-carbon single bond has a length of 154 pm, while a typical double bond and triple bonds are 134 pm and 120 pm, respectively.

We can see that
Ethane has a single bond between carbon and carbon.
In benzene, the carbon-carbon bond lengths are in resonance due to its aromatic nature, so they have bond length between single bond and double bond as it exhibits partial double bond character.
In ethene, there is a double bond between carbon and carbon.
In ethyne, there is a triple bond between carbon and carbon.
Thus, Single bond > Partial double bond > Double bond > Triple bond.
Therefore, we get the correct following order:
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]
So, the correct option is B.
Note: Partial double bond character is exhibited by molecules having resonance structures where both single bonds and double bonds are exhibited by the molecule. These molecules have bond lengths more than single bonds but less than double bonds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




