
The total number of contributing structures showing hyperconjugation (involving $C - H$ bonds) for the following carbocation is:
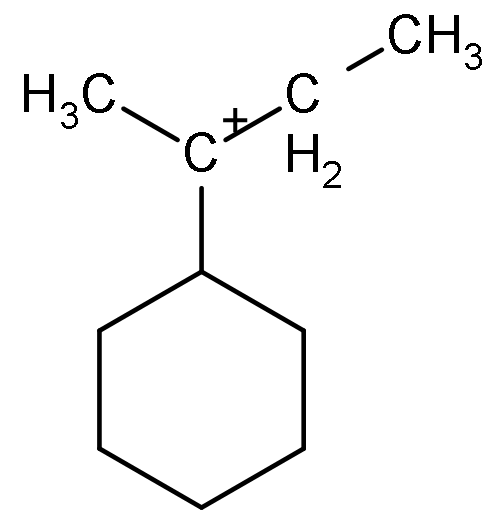
(A) 4
(B) 5
(C) 6
(D) 7
Answer
233.1k+ views
Hint: For this problem, we have to count the total number of alpha hydrogens that are attached to the alpha carbons because the number of hyperconjugation structures is directly proportional to the number of alpha hydrogen present in the structure.
Complete step by step solution:
- In the given question, we have to explain the total number of contributing structures which will show hyperconjugation.
- Now, firstly we should know about the hyperconjugation as it is a process in which the delocalisation of the electron will take place.
- The delocalisation of the electron takes place between the sigma or single bond and pi bond or non-bonding lone pair.
- So, to calculate the total number of conjugating structures, the compound must have the alpha hydrogen.
- Now, alpha hydrogen is the hydrogen which is directly attached to the alpha carbon and alpha carbon is the carbon which is directly attached to the functional group or carbocation.
- Also, we know that the total number of hyperconjugation structures is directly proportional to the total number of alpha hydrogen atoms present in the structure.
- So, in the given compound as we can see that there is three alpha carbon that is attached to the carbocation directly.
- And out of the left alpha carbon has three alpha hydrogen, right alpha carbon has two alpha hydrogen and the alpha carbon that is present below the carbocation has one alpha carbocation.
- So, the total number of alpha-hydrogen will be $3 + 2 + 1 = 5$.
Therefore, option (B) is the correct answer.
Note: Hyperconjugation is different from that of the resonance because in hyperconjugation the delocalisation of the sigma and non-bonding electron takes place whereas in resonance the delocalisation of the pi electrons takes place only.
Complete step by step solution:
- In the given question, we have to explain the total number of contributing structures which will show hyperconjugation.
- Now, firstly we should know about the hyperconjugation as it is a process in which the delocalisation of the electron will take place.
- The delocalisation of the electron takes place between the sigma or single bond and pi bond or non-bonding lone pair.
- So, to calculate the total number of conjugating structures, the compound must have the alpha hydrogen.
- Now, alpha hydrogen is the hydrogen which is directly attached to the alpha carbon and alpha carbon is the carbon which is directly attached to the functional group or carbocation.
- Also, we know that the total number of hyperconjugation structures is directly proportional to the total number of alpha hydrogen atoms present in the structure.
- So, in the given compound as we can see that there is three alpha carbon that is attached to the carbocation directly.
- And out of the left alpha carbon has three alpha hydrogen, right alpha carbon has two alpha hydrogen and the alpha carbon that is present below the carbocation has one alpha carbocation.
- So, the total number of alpha-hydrogen will be $3 + 2 + 1 = 5$.
Therefore, option (B) is the correct answer.
Note: Hyperconjugation is different from that of the resonance because in hyperconjugation the delocalisation of the sigma and non-bonding electron takes place whereas in resonance the delocalisation of the pi electrons takes place only.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




