
The type of elements present in group 3 to 12[IB to VIIB and VIII] are:
A. Alkali
B. Alkaline
C. Transition
D. halogen
Answer
232.8k+ views
Hint: According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 replacing the older notation of groups IA… VIIA, VIII, 1B and 0. This is called a long form of periodic table.
Complete step-by-step answer:
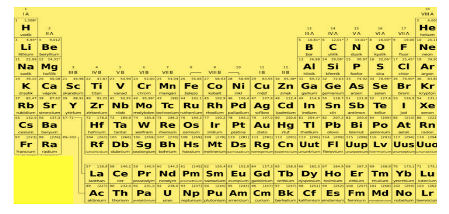
Elements in the same column of the periodic table have the same number of valence electrons in their outer energy level. This leads to their similar properties. All the elements in group 1(IA) have just one valence electron and therefore they are highly reactive. All the other elements in group 1 except hydrogen are alkali metals.
The alkaline earth metals include all the elements in group 2(IIA). These have just two valence electrons, they are reactive but less than alkali metals. Groups 3-12(IB to VIIB and VIII) of the periodic table contain transition metals. They have more valence electrons and are less reactive than the first two groups.
Groups 13-16 each contain one or more metalloids, group 13(IIIA) is boron group(boron is a metalloid). Group 14(IVA) is carbon group, group 15(VA) is nitrogen group and group 16(VIA) is oxygen group. These groups have nonmetals and metalloids as their elements.
Elements in group 17(VIIA) belong to the halogen family. They are very reactive non-metals with seven electrons in their valence shell. Group 18(VIIIA) elements are also non-metals and called noble gases. Their outer energy level is full, hence they are very stable and least reactive.
Hence, the correct option is (C).
Note: This modern periodic table states that the physical and chemical properties of the elements are periodic functions of their atomic numbers. But as per old periodic table by Mendeleev, it was a periodic function of atomic masses. It was rejected because he was not able to locate hydrogen and isotopes of elements which were found later on.
Complete step-by-step answer:
Elements in the same column of the periodic table have the same number of valence electrons in their outer energy level. This leads to their similar properties. All the elements in group 1(IA) have just one valence electron and therefore they are highly reactive. All the other elements in group 1 except hydrogen are alkali metals.
The alkaline earth metals include all the elements in group 2(IIA). These have just two valence electrons, they are reactive but less than alkali metals. Groups 3-12(IB to VIIB and VIII) of the periodic table contain transition metals. They have more valence electrons and are less reactive than the first two groups.
Groups 13-16 each contain one or more metalloids, group 13(IIIA) is boron group(boron is a metalloid). Group 14(IVA) is carbon group, group 15(VA) is nitrogen group and group 16(VIA) is oxygen group. These groups have nonmetals and metalloids as their elements.
Elements in group 17(VIIA) belong to the halogen family. They are very reactive non-metals with seven electrons in their valence shell. Group 18(VIIIA) elements are also non-metals and called noble gases. Their outer energy level is full, hence they are very stable and least reactive.

Hence, the correct option is (C).
Note: This modern periodic table states that the physical and chemical properties of the elements are periodic functions of their atomic numbers. But as per old periodic table by Mendeleev, it was a periodic function of atomic masses. It was rejected because he was not able to locate hydrogen and isotopes of elements which were found later on.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




