
Which of the following compounds has an isopropyl group?
A.2 – methyl pentane
B.2, 2, 3 – trimethylpentane
C.2, 2, 3, 3 – tetramethyl pentane
D.All of these
Answer
232.8k+ views
Hint: While considering the propyl group, the carbon atoms at the position 1 and 2 have the capacity to form a carbocation. If the carbocation is formed at position 1, then the propyl group is named as n – propyl. On the other hand, if the carbocation is formed at position 2, then the propyl group is known as isopropyl.
Complete Step-by-Step Answer:
Before we move towards the solution of this question, let us first understand some important basic concepts.
Alkanes are aliphatic organic compounds which have a minimum of 1 and maximum ‘n’ number of carbon atoms. Also, only sigma bonds are present in alkanes. This means that alkanes are formed only with single bonds, and not double or triple bonds. When we make an alkane with 3 carbon atoms, then the organic compound is known as propane. The chemical formula for propane can be given as \[{C_3}{H_8}\] . Propane loses one hydrogen atom to form the propyl group. This propyl group takes place in various reactions and acts as a site for substitution, addition, or elimination. It can be basically explained as a three-carbon alkane with a carbocation.
Now, this propyl group may have one terminal or one internal carbocation site. In simpler terms, the carbon atoms at the position 1 and 2 have the capacity to form a carbocation. If the carbocation is formed at position 1, then the propyl group is named as n – propyl. On the other hand, if the carbocation is formed at position 2, then the propyl group is known as isopropyl. The molecular structure of isopropyl group can be shown as follows:

Now, moving back to the question, we can identify the presence of isopropyl groups by observing the molecular structures of the given compounds.
A.2 – methyl pentane
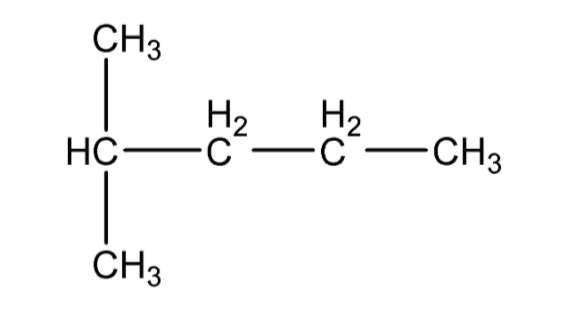
B.2, 2, 3 – trimethylpentane

C.2, 2, 3, 3 – tetramethyl pentane
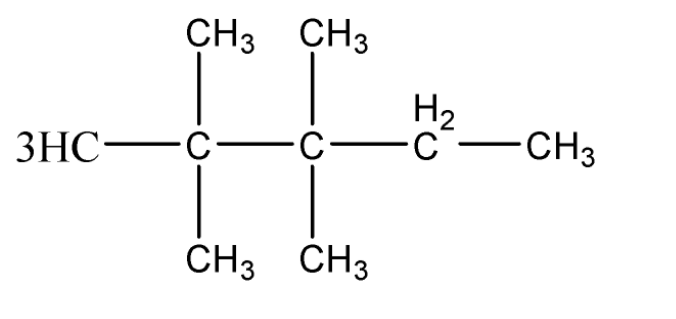
Hence, we can observe that 2 – methyl pentane is the only compound that has an isopropyl group present.
Hence, Option A is the correct option
Note: We know that higher the degree of the carbocation, higher is the stability of the compound. Hence \[2^\circ \] carbocation is more stable than \[1^\circ \] carbocation. This means that the isopropyl group is more stable than n – propyl group.
Complete Step-by-Step Answer:
Before we move towards the solution of this question, let us first understand some important basic concepts.
Alkanes are aliphatic organic compounds which have a minimum of 1 and maximum ‘n’ number of carbon atoms. Also, only sigma bonds are present in alkanes. This means that alkanes are formed only with single bonds, and not double or triple bonds. When we make an alkane with 3 carbon atoms, then the organic compound is known as propane. The chemical formula for propane can be given as \[{C_3}{H_8}\] . Propane loses one hydrogen atom to form the propyl group. This propyl group takes place in various reactions and acts as a site for substitution, addition, or elimination. It can be basically explained as a three-carbon alkane with a carbocation.
Now, this propyl group may have one terminal or one internal carbocation site. In simpler terms, the carbon atoms at the position 1 and 2 have the capacity to form a carbocation. If the carbocation is formed at position 1, then the propyl group is named as n – propyl. On the other hand, if the carbocation is formed at position 2, then the propyl group is known as isopropyl. The molecular structure of isopropyl group can be shown as follows:

Now, moving back to the question, we can identify the presence of isopropyl groups by observing the molecular structures of the given compounds.
A.2 – methyl pentane

B.2, 2, 3 – trimethylpentane

C.2, 2, 3, 3 – tetramethyl pentane

Hence, we can observe that 2 – methyl pentane is the only compound that has an isopropyl group present.
Hence, Option A is the correct option
Note: We know that higher the degree of the carbocation, higher is the stability of the compound. Hence \[2^\circ \] carbocation is more stable than \[1^\circ \] carbocation. This means that the isopropyl group is more stable than n – propyl group.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




