
Which of the following meet the requirements of Huckel Rule?
(A) Naphthalene
(B) Cyclohexane
(C) 1,3,5,7 - Cyclooctatetraene
(D) 1,3 - Cyclobutadiene
Answer
233.1k+ views
Hint: In organic chemistry Huckel rule estimates whether a planar ring molecule will have aromatic properties or not. We need to analyse the structures of the compounds given in the options.
Complete step by step answer:
> Huckel rule states that in order to be aromatic a molecule must have a certain number of \[\text{ }\!\!\pi\!\!\text{ }\] electrons (that is electrons with pi bonds or lone pairs within p orbitals) within a closed loop of parallel, adjacent p orbitals.
To be more specific we can say that, if a cyclic, planar molecule has \[\text{4n+2 }\!\!\pi\!\!\text{ }\] electrons, it is an aromatic molecule. Here n is an integer which possess the value 0,1,2…etc.
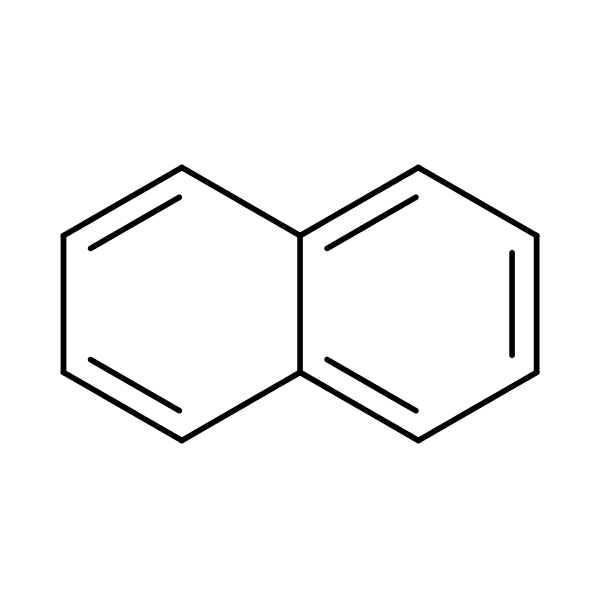
> The above is the structure of Naphthalene. From the diagram, we can see that there are 5
double bonds. The number of 10 pi electrons is 10.
So, the value of \[n\text{ }=\text{ }4\times n\text{ }+\text{ }2\text{ }=\text{ }10\]
So, \[\text{ }\!\!~\!\!\text{ n = 2}\] (which is an integer)
So, we can say that naphthalene meets the requirements of the Huckel Rule.
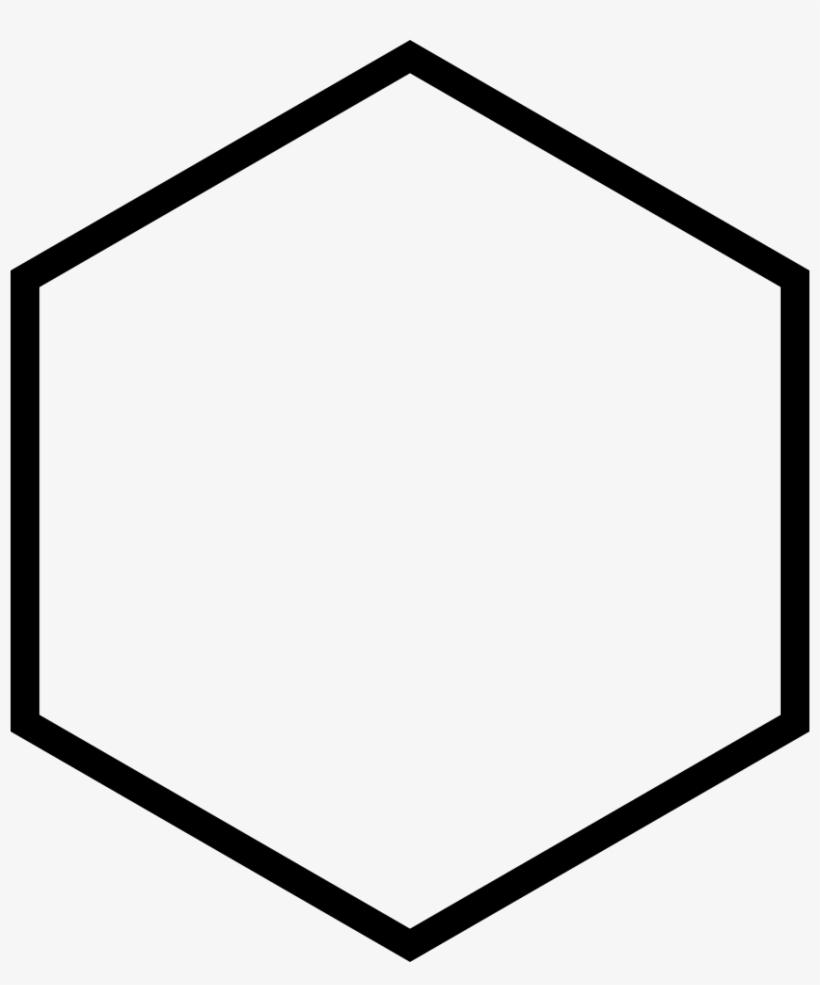
> The above is the structure of cyclohexane. Aromaticity requires cyclic conjugation that is
the alteration of single and double bonds or lone pairs and single bonds and double bonds
around the ring. Cyclohexane has only one double bond so it is not conjugated. Therefore, it cannot be automatic.
So we can say that cyclohexane does not follow the requirements of Huckel Rule.
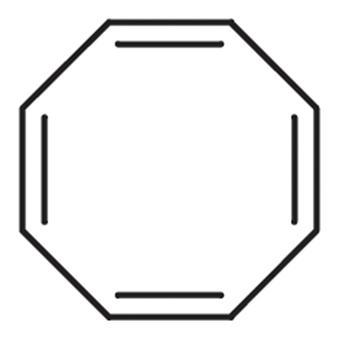
> The above is the diagram of 1,3,5,7 - Cyclooctatetraene. From the structure we can figure out that it is not planar in structure. So cannot be aromatic in nature. So we can say that 1,3,5,7 - Cyclooctatetraene does not follow the requirements of Huckel Rule.

> The above is the diagram of 1,3 - Cyclobutadiene. From the diagram it is clearer than the number of \[\text{ }\!\!\pi\!\!\text{ }\] electrons it is having is even in number and not odd. Therefore, it does not satisfy the \[\text{4n+2 }\!\!\pi\!\!\text{ }\] electrons criteria of the Huckel Rule. So we can say that 1,3 - Cyclobutadiene does not follow the requirements of Huckel Rule.
Therefore, we conclude that Option A is the correct answer.
Note: Criteria for aromaticity:
i) It must have a planar ring.
ii) A continuous chain of unhybridized p orbitals.
iii) Odd number of delocalized electron pairs in the system.
Complete step by step answer:
> Huckel rule states that in order to be aromatic a molecule must have a certain number of \[\text{ }\!\!\pi\!\!\text{ }\] electrons (that is electrons with pi bonds or lone pairs within p orbitals) within a closed loop of parallel, adjacent p orbitals.
To be more specific we can say that, if a cyclic, planar molecule has \[\text{4n+2 }\!\!\pi\!\!\text{ }\] electrons, it is an aromatic molecule. Here n is an integer which possess the value 0,1,2…etc.

> The above is the structure of Naphthalene. From the diagram, we can see that there are 5
double bonds. The number of 10 pi electrons is 10.
So, the value of \[n\text{ }=\text{ }4\times n\text{ }+\text{ }2\text{ }=\text{ }10\]
So, \[\text{ }\!\!~\!\!\text{ n = 2}\] (which is an integer)
So, we can say that naphthalene meets the requirements of the Huckel Rule.

> The above is the structure of cyclohexane. Aromaticity requires cyclic conjugation that is
the alteration of single and double bonds or lone pairs and single bonds and double bonds
around the ring. Cyclohexane has only one double bond so it is not conjugated. Therefore, it cannot be automatic.
So we can say that cyclohexane does not follow the requirements of Huckel Rule.

> The above is the diagram of 1,3,5,7 - Cyclooctatetraene. From the structure we can figure out that it is not planar in structure. So cannot be aromatic in nature. So we can say that 1,3,5,7 - Cyclooctatetraene does not follow the requirements of Huckel Rule.

> The above is the diagram of 1,3 - Cyclobutadiene. From the diagram it is clearer than the number of \[\text{ }\!\!\pi\!\!\text{ }\] electrons it is having is even in number and not odd. Therefore, it does not satisfy the \[\text{4n+2 }\!\!\pi\!\!\text{ }\] electrons criteria of the Huckel Rule. So we can say that 1,3 - Cyclobutadiene does not follow the requirements of Huckel Rule.
Therefore, we conclude that Option A is the correct answer.
Note: Criteria for aromaticity:
i) It must have a planar ring.
ii) A continuous chain of unhybridized p orbitals.
iii) Odd number of delocalized electron pairs in the system.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
Understanding Average and RMS Value in Electrical Circuits

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Understanding Elastic Collisions in Two Dimensions

For pure water A pH increases while pOH decreases with class 11 chemistry JEE_Main

Which of the following is most stable A Sn2+ B Ge2+ class 11 chemistry JEE_Main

Other Pages
NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)

An ideal gas is at pressure P and temperature T in class 11 chemistry JEE_Main

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding Collisions: Types and Examples for Students

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages




