
Which plastic is commonly used as carry bags?
A. Polythene
B. Polyester
C. PET
D. Acrylic
Answer
232.8k+ views
Hint: This plastic is made of straight molecule chains that branch very little, staying linear from beginning to end. This linear structure creates a very strong material, which is why the common grocery bag is light yet can hold many times its own weight without tearing.
Step by step explanation:
Polythene or polyethylene is a thermoplastic polymer consisting of long hydrocarbon chains. Polyethylene or polythene is the most common plastic. Its primary use is in packaging (plastic bags, plastic films, containers including bottles, etc.). So option (A) is correct.
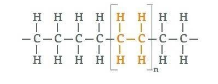
Polyester is a synthetic fabric that's usually derived from petroleum. As it is costly so it is not used in carry bags. So option (B) is incorrect.
PET, which stands for polyethylene terephthalate, is a form of polyester (just like the clothing fabric). It is extruded or molded into plastic bottles and containers for packaging foods and beverages, personal care products, and many other consumer products. So option (C) is also incorrect.
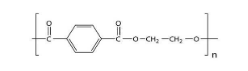
Acrylic is a transparent plastic that has gained widespread use because it's ability to replace the glass. But The melting point for acrylic plastic is 160 degrees C, so they cannot withstand extreme temperatures. So option (D) is also incorrect.
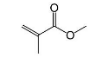
So option (A) is correct.
Note: Polythene has low strength and hardness, but is very ductile and has good impact strength; it will stretch rather than break. Polyethylene is water resistant and durable, so it is longer lasting when exposed to the elements compared to other polymers. Some disadvantages of polythene bags:
- High thermal expansion.
- Poor weathering resistance.
- Subject to stress cracking.
- Difficult to bond.
- Flammable.
- Poor temperature capability.
Step by step explanation:
Polythene or polyethylene is a thermoplastic polymer consisting of long hydrocarbon chains. Polyethylene or polythene is the most common plastic. Its primary use is in packaging (plastic bags, plastic films, containers including bottles, etc.). So option (A) is correct.

Polyester is a synthetic fabric that's usually derived from petroleum. As it is costly so it is not used in carry bags. So option (B) is incorrect.
PET, which stands for polyethylene terephthalate, is a form of polyester (just like the clothing fabric). It is extruded or molded into plastic bottles and containers for packaging foods and beverages, personal care products, and many other consumer products. So option (C) is also incorrect.

Acrylic is a transparent plastic that has gained widespread use because it's ability to replace the glass. But The melting point for acrylic plastic is 160 degrees C, so they cannot withstand extreme temperatures. So option (D) is also incorrect.

So option (A) is correct.
Note: Polythene has low strength and hardness, but is very ductile and has good impact strength; it will stretch rather than break. Polyethylene is water resistant and durable, so it is longer lasting when exposed to the elements compared to other polymers. Some disadvantages of polythene bags:
- High thermal expansion.
- Poor weathering resistance.
- Subject to stress cracking.
- Difficult to bond.
- Flammable.
- Poor temperature capability.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




