
(1) Draw lewis structure of ${{O}_{3}}$ and $HN{{O}_{3}}$.
(2) Write the formula for iron (III) sulphate and Nickel (II) sulphate.
Answer
576.9k+ views
Hint:. (1) A structure is a graphic representation of the electron distribution around the atoms is known as Lewis structure. Lewis structure is drawn to predict the number and type of bonds that may be formed around an atom. A lewis structure is also used to predict the geometry of a molecule.
(2) A way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, number, and sometimes symbols such as parentheses, dashes, brackets, commas and (+) or (-) signs is called a chemical formula.
Complete step by step answer:
(1) For drawing the lewis structure, we need to follow the following steps-
(i) The first step in drawing the Lewis structure involves the number of valence electrons in the molecule or ion. For a neutral molecule, it is simply the sum of all the valence electrons. If the molecule carries an electrical charge, we add one electron for each negative charge and subtract an electron for each positive charge.
(ii) The second step in drawing the Lewis structure involves deciding which atoms in the molecule are connected by covalent atom to which atom. The formula of the compound gives us a hint about the skeleton structure of the compound or molecule.
(iii) The third step is assuming that the skeleton structure of the molecule is held by covalent bonds. The valence electrons are divided into two categories- bonding electrons and non-bonding electrons. As the covalent bond is formed by two electrons, we can calculate the total number of valence electrons for each bond in the skeleton structure.
(iv) Last step is completing the octet for the central atom with the remaining electrons. If there are any bonds left over, create double bonds with lone pairs on outside atoms. This will complete the Lewis dot structure for the molecule.
-Following the above steps, we will now draw the Lewis dot structure of ${{O}_{3}}$ and $HN{{O}_{3}}$-
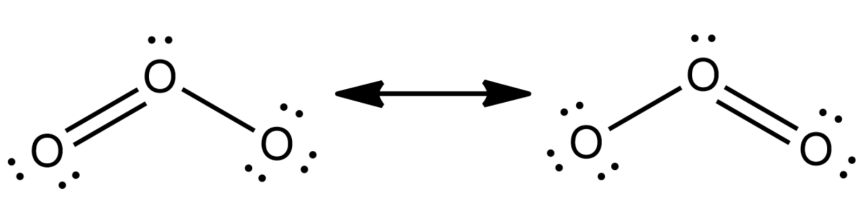
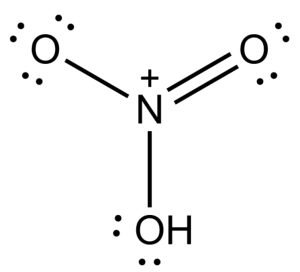
(2) In order to write the chemical structure we need to follow the following steps-
(i) First, we need to decide the type of bond present between the atoms. If the prefixes are used, then it is a covalent bond. In other cases, if there are no prefixes then it is an ionic bond.
(ii) In the second step, we will write down the symbol of the polyatomic ion or the element.
(iii) In the third step, if the prefix was used, we will add a subscript. We will add a subscript by balancing the charge.
Following the above steps, we will write the formula of iron (III) sulphate and Nickel (II) sulphate as-
$F{{e}_{2}}{{(S{{O}_{4}})}_{3}}$ ; $NiS{{O}_{4}}$
Note: (1) While Lewis structures are very useful in finding valency, oxidation states, bonding and predicting geometries, there are many exceptions to these rules in the real world. Atoms seek to become fully or partially fill their valence shell. However, atoms can or cannot form molecules which are not ideally stable but do exist. In some cases, the central atom can also form more than other atoms connected to it. In some cases, the number of valence electrons can exceed eight, especially in higher atomic numbers. Lewis structures are helpful for lighter elements but less useful for lanthanides and actinides.
(2) There are some tricks which you can keep in mind while writing the chemical formula of a chemical compound-
(i) For most of the covalent compounds the clue is in the name like carbon monoxide means carbon and one (mono) oxygen atom.
(ii) A handful of exceptions have special names such as ammonia $(N{{H}_{3}})$, water $({{H}_{2}}O)$ , methane $(C{{H}_{4}})$ , ethane $({{C}_{2}}{{H}_{6}})$ , propane $({{C}_{3}}{{H}_{8}})$ and butane $({{C}_{4}}{{H}_{10}})$ .
(iii) Seven elements exist as molecules (diatomic) which are hydrogen, nitrogen. Oxygen, fluorine, chlorine, bromine, and iodine.
(iv) Compounds like atoms, don’t have an overall charge, for example, sodium chloride $N{{a}^{+}}$ and $C{{l}^{-}}$ becomes and NaCl.
(v) Some ionic compounds involve complexions like hydroxide $(O{{H}^{-}})$, sulfate $(S{{O}_{4}}^{2-})$ , nitrate $(N{{O}_{3}}^{-})$ , carbonate $(C{{O}_{3}}^{2-})$ and ammonium $(N{{H}_{4}}^{+})$ .
(2) A way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, number, and sometimes symbols such as parentheses, dashes, brackets, commas and (+) or (-) signs is called a chemical formula.
Complete step by step answer:
(1) For drawing the lewis structure, we need to follow the following steps-
(i) The first step in drawing the Lewis structure involves the number of valence electrons in the molecule or ion. For a neutral molecule, it is simply the sum of all the valence electrons. If the molecule carries an electrical charge, we add one electron for each negative charge and subtract an electron for each positive charge.
(ii) The second step in drawing the Lewis structure involves deciding which atoms in the molecule are connected by covalent atom to which atom. The formula of the compound gives us a hint about the skeleton structure of the compound or molecule.
(iii) The third step is assuming that the skeleton structure of the molecule is held by covalent bonds. The valence electrons are divided into two categories- bonding electrons and non-bonding electrons. As the covalent bond is formed by two electrons, we can calculate the total number of valence electrons for each bond in the skeleton structure.
(iv) Last step is completing the octet for the central atom with the remaining electrons. If there are any bonds left over, create double bonds with lone pairs on outside atoms. This will complete the Lewis dot structure for the molecule.
-Following the above steps, we will now draw the Lewis dot structure of ${{O}_{3}}$ and $HN{{O}_{3}}$-


(2) In order to write the chemical structure we need to follow the following steps-
(i) First, we need to decide the type of bond present between the atoms. If the prefixes are used, then it is a covalent bond. In other cases, if there are no prefixes then it is an ionic bond.
(ii) In the second step, we will write down the symbol of the polyatomic ion or the element.
(iii) In the third step, if the prefix was used, we will add a subscript. We will add a subscript by balancing the charge.
Following the above steps, we will write the formula of iron (III) sulphate and Nickel (II) sulphate as-
$F{{e}_{2}}{{(S{{O}_{4}})}_{3}}$ ; $NiS{{O}_{4}}$
Note: (1) While Lewis structures are very useful in finding valency, oxidation states, bonding and predicting geometries, there are many exceptions to these rules in the real world. Atoms seek to become fully or partially fill their valence shell. However, atoms can or cannot form molecules which are not ideally stable but do exist. In some cases, the central atom can also form more than other atoms connected to it. In some cases, the number of valence electrons can exceed eight, especially in higher atomic numbers. Lewis structures are helpful for lighter elements but less useful for lanthanides and actinides.
(2) There are some tricks which you can keep in mind while writing the chemical formula of a chemical compound-
(i) For most of the covalent compounds the clue is in the name like carbon monoxide means carbon and one (mono) oxygen atom.
(ii) A handful of exceptions have special names such as ammonia $(N{{H}_{3}})$, water $({{H}_{2}}O)$ , methane $(C{{H}_{4}})$ , ethane $({{C}_{2}}{{H}_{6}})$ , propane $({{C}_{3}}{{H}_{8}})$ and butane $({{C}_{4}}{{H}_{10}})$ .
(iii) Seven elements exist as molecules (diatomic) which are hydrogen, nitrogen. Oxygen, fluorine, chlorine, bromine, and iodine.
(iv) Compounds like atoms, don’t have an overall charge, for example, sodium chloride $N{{a}^{+}}$ and $C{{l}^{-}}$ becomes and NaCl.
(v) Some ionic compounds involve complexions like hydroxide $(O{{H}^{-}})$, sulfate $(S{{O}_{4}}^{2-})$ , nitrate $(N{{O}_{3}}^{-})$ , carbonate $(C{{O}_{3}}^{2-})$ and ammonium $(N{{H}_{4}}^{+})$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




