
\[4\]-Hydroxy- \[4\]-methyl pentanal on heating with excess of methanol in the presence of an acid catalyst followed by dehydration of the product gives
A)
B)
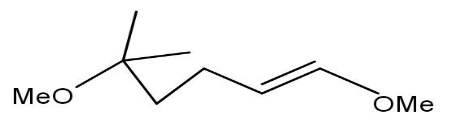
C)
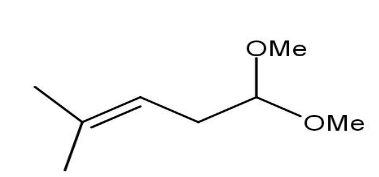
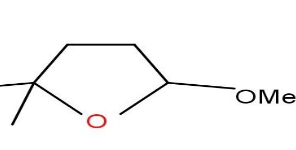
D)
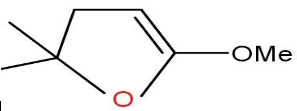




Answer
498k+ views
Hint: The carbonyl compounds when heated with excess methanol then two methoxy groups were inserted on the carbonyl carbon.
When alcohols also known as hydroxy compounds undergo dehydration, they form alkenes.
Complete answer: Given compound is \[4 - hydroxy{\text{ }} - 4 - methylpentanal.\]
The parent chain is pentanal, it has five carbon atoms. There is a substitution at fourth carbon.
The numbering should be started from aldehydic carbon.
There are both methyl and hydroxyl groups on \[{4^{th}}\] carbon.
When given compound treats with excess methanol, then the aldehydic carbon converts into the carbon containing methoxy and hydroxyl groups.
As it is treated with excess methanol, the hydroxyl group also converts into methoxy group.
\[{\left( {C{H_3}} \right)_2}C\left( {OH} \right)C{H_2}C{H_2}CH\left( {OH} \right)\left( {C{H_3}} \right) \to {\left( {C{H_3}} \right)_2}C\left( {OH} \right)C{H_2}C{H_2}CH{\left( {OC{H_3}} \right)_2}\]
Now the above product, treated with an acid catalyst, undergoes dehydration.
Dehydration is defined as the loss of water molecules.
The hydroxyl group on \[{4^{th}}\] carbon and hydrogen on \[{3^{rd}}\] carbon ate lost as a water molecule.
Thus, the \[{4^{th}}\] carbon gets positive charge, and the third carbon gets negative charge.
Thereby forms an alkene.
Alkene is an unsaturated organic compound with the presence of a double bond.
Thus, when the given compound undergoes heating with excess methanol in the presence of acid catalyst followed by dehydration it forms a compound namely\[1,1 - dimethoxy - 4 - methyl{\text{ }}3 - pentene\].
Option A will not be formed as the methylation cannot be done on \[{1^{st}}\] carbon.
Option C and D are also not formed as the compound does not undergo cyclisation.
Thus, option B is the correct one.
Note:
The methylation should be done on the aldehydic carbon only. At first one methoxy group attacks on aldehydic carbon, and then due to excess methanol the hydroxyl group can also convert to methoxy group.
The water molecule can be lost from the neighboring carbon atoms only.
When alcohols also known as hydroxy compounds undergo dehydration, they form alkenes.
Complete answer: Given compound is \[4 - hydroxy{\text{ }} - 4 - methylpentanal.\]
The parent chain is pentanal, it has five carbon atoms. There is a substitution at fourth carbon.
The numbering should be started from aldehydic carbon.
There are both methyl and hydroxyl groups on \[{4^{th}}\] carbon.
When given compound treats with excess methanol, then the aldehydic carbon converts into the carbon containing methoxy and hydroxyl groups.
As it is treated with excess methanol, the hydroxyl group also converts into methoxy group.
\[{\left( {C{H_3}} \right)_2}C\left( {OH} \right)C{H_2}C{H_2}CH\left( {OH} \right)\left( {C{H_3}} \right) \to {\left( {C{H_3}} \right)_2}C\left( {OH} \right)C{H_2}C{H_2}CH{\left( {OC{H_3}} \right)_2}\]
Now the above product, treated with an acid catalyst, undergoes dehydration.
Dehydration is defined as the loss of water molecules.
The hydroxyl group on \[{4^{th}}\] carbon and hydrogen on \[{3^{rd}}\] carbon ate lost as a water molecule.
Thus, the \[{4^{th}}\] carbon gets positive charge, and the third carbon gets negative charge.
Thereby forms an alkene.
Alkene is an unsaturated organic compound with the presence of a double bond.
Thus, when the given compound undergoes heating with excess methanol in the presence of acid catalyst followed by dehydration it forms a compound namely\[1,1 - dimethoxy - 4 - methyl{\text{ }}3 - pentene\].
Option A will not be formed as the methylation cannot be done on \[{1^{st}}\] carbon.
Option C and D are also not formed as the compound does not undergo cyclisation.
Thus, option B is the correct one.
Note:
The methylation should be done on the aldehydic carbon only. At first one methoxy group attacks on aldehydic carbon, and then due to excess methanol the hydroxyl group can also convert to methoxy group.
The water molecule can be lost from the neighboring carbon atoms only.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




